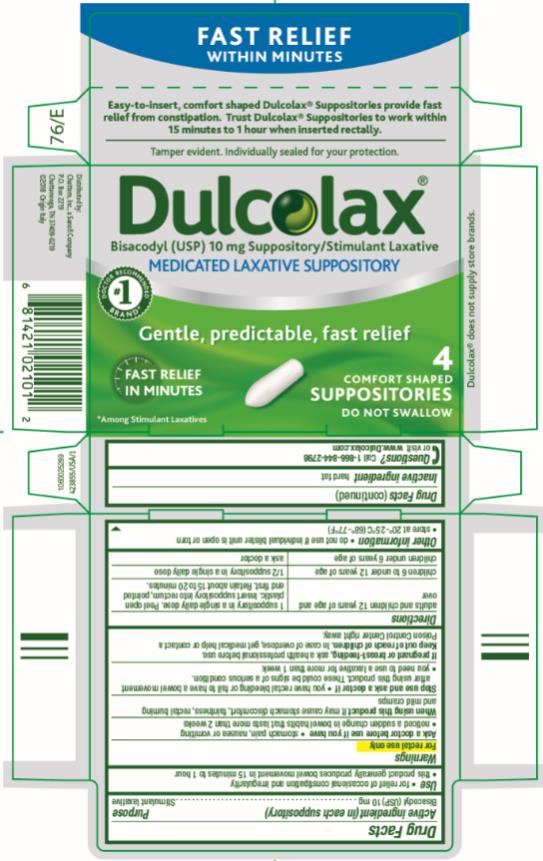
Dulcolax®
Medicated Laxative Suppositories
Drug Facts
Active ingredient (in each suppository)
Bisacodyl (USP) 10 mg
Purpose
Stimulant laxative
Use
- for relief of occasional constipation and irregularity
- this product generally produces bowel movement in 15 minutes to 1 hour
Warnings
For rectal use only
Ask a doctor before use if you have
- stomach pain, nausea or vomiting
- noticed a sudden change in bowel habits that lasts more than 2 weeks
When using this product
it may cause stomach discomfort, faintness, rectal burning and mild cramps
Stop use and ask a doctor if
- you have rectal bleeding or fail to have a bowel movement after using this product. These could be signs of a serious condition.
- you need to use a laxative for more than 1 week
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
adults and children 12 years of age and over
1 suppository in a single daily dose. Peel open plastic. Insert suppository into rectum, pointed end first. Retain about 15 to 20 minutes.
children 6 to under 12 years of age
½ suppository in a single daily dose
children under 6 years of age
ask a doctor
Other information
- do not use if individual blister unit is open or torn
- store at 20°-25°C (68°-77°F)
Inactive ingredient
hard fat
Questions?
Call 1-866-844-2798 or visit www.Dulcolax.com
Keep carton as it contains important product information.
PRINCIPAL DISPLAY PANEL
Dulcolax
Bisacodyl (USP) 10 mg Suppository/ Stimulant Laxative
MEDICATED LAXATIVE SUPPOSITORY
4
COMFORT SHAPED
SUPPOSITORIES
DO NOT SWALLOW
Chattem, Inc.
