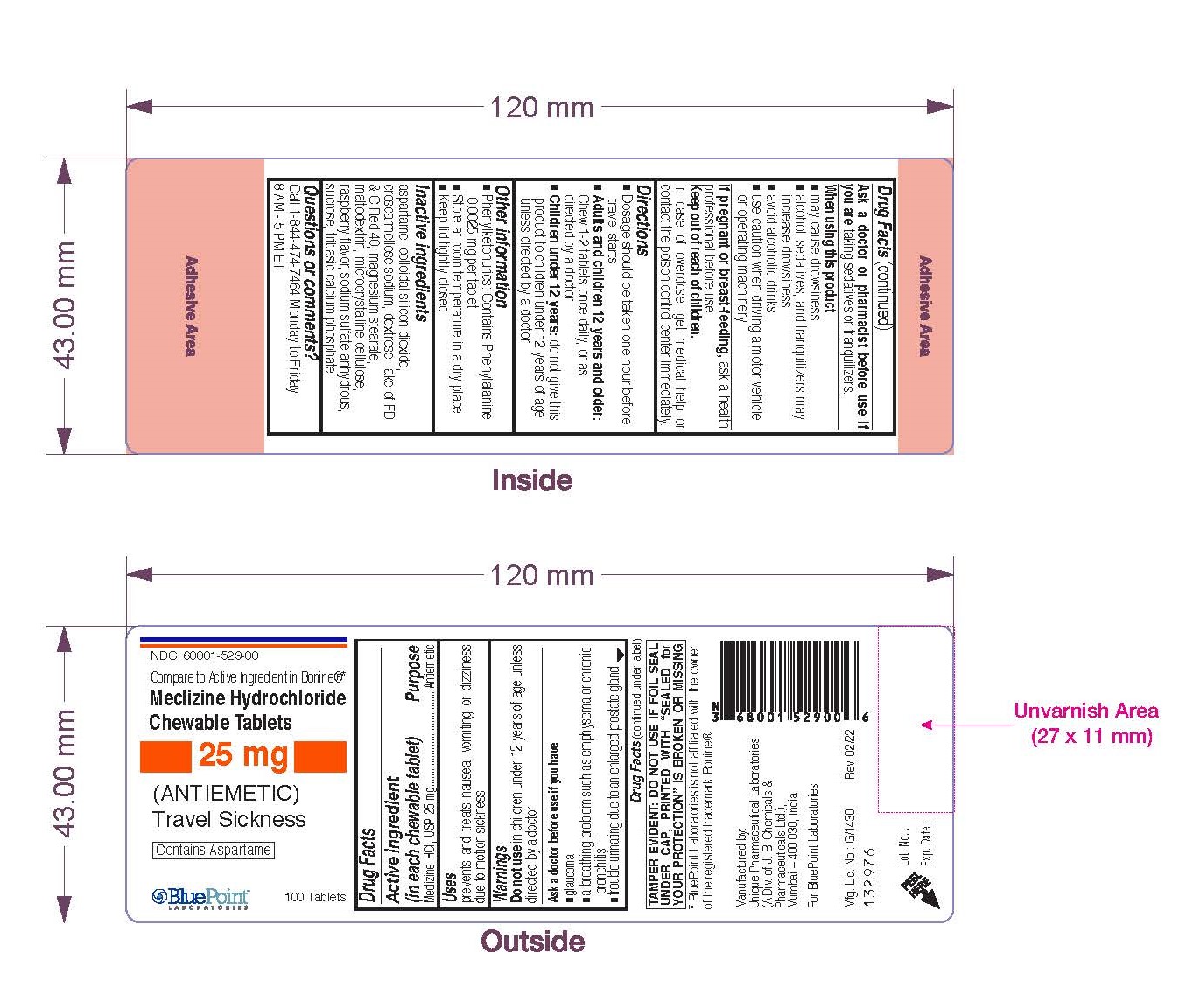
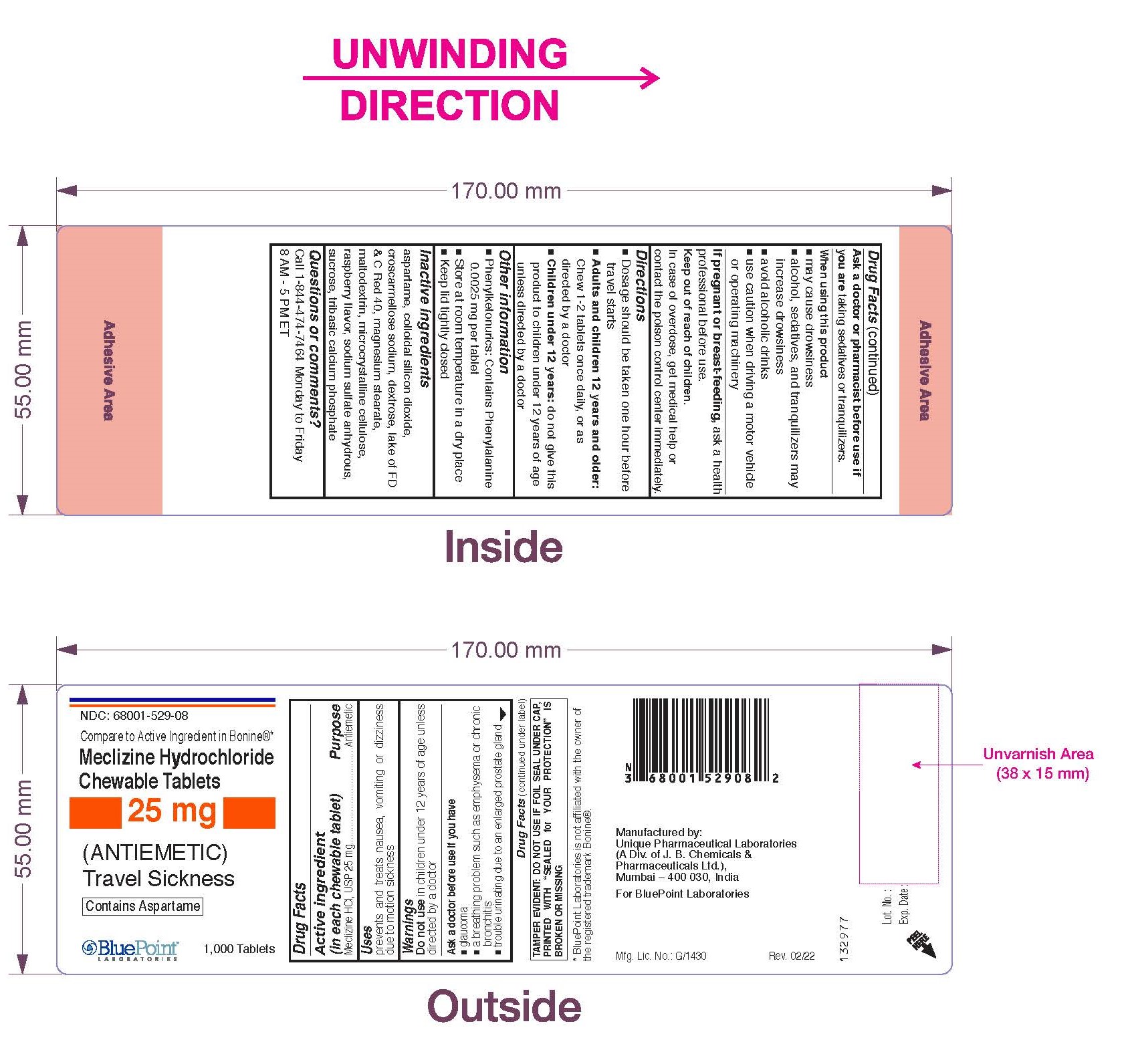
Warnings
Do not use in children under 12 years of age unless directed by a doctor
Ask a doctor before use if you have
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- trouble urinating due to an enlarged prostate gland
When using this product
- may cause drowsiness
- alcohol, sedatives, and tranquilizers may increase drowsiness
- avoid alcoholic drinks
- use caution when driving a motor vehicle or operating machinery
Keep out of reach of children.
In case of overdose, get medical help or contact the Poison Control Center immediately
Directions
- Dosage should be taken one hour before travel starts
- Adults and children 12 years and older: Chew 1-2 tablets once daily, or as directed by a doctor
- Children under 12 years: do not give this product to children under 12 years of age unless directed by a doctor
Other Information
- Phenylketonurics: Contains Phenylalanine 0.0025 mg per tablet
- Store at room temperature in a dry place
- Keep lid tightly closed