Label: SAFETUSSIN PM NIGHTTIME COUGH RELIEF- dextromethorphan hbr, doxylamine succinate liquid
- NDC Code(s): 55505-221-33, 55505-221-36
- Packager: Kramer Laboratories
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated June 28, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- Uses
-
Warnings
- Do not useif you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product
- .to make a child sleep.
- Ask a doctor before use if you have
- ASK DOCTOR/PHARMACIST
- When using this product
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information:
- INACTIVE INGREDIENT
-
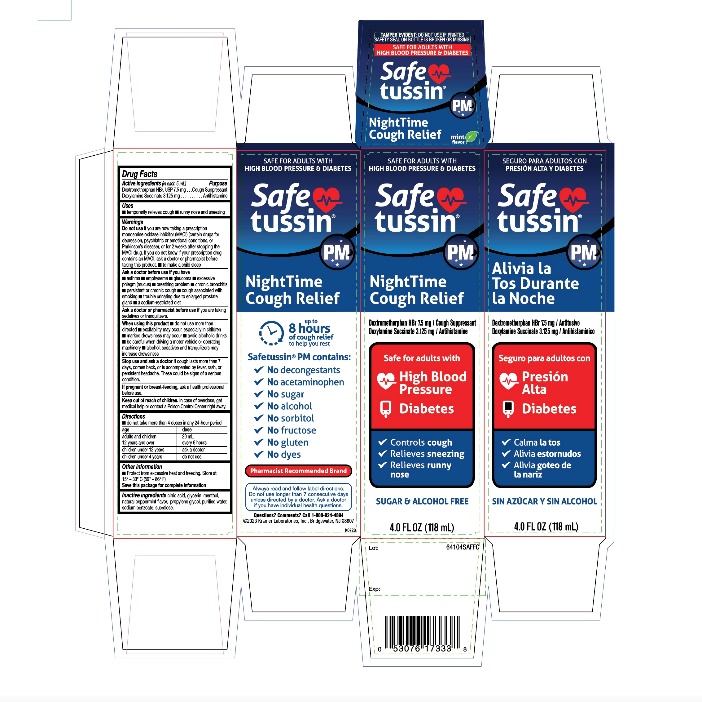
PRINCIPAL DISPLAY PANEL
SAFE FOR ADULTS WITH
HIGH BLOOD PRESSURE & DIABETES
Safetussin ®PM
NightTime
Cough Relief
Dextromethorphan HBr 7.5 mg / Cough SuppressantDoxylamine Succinate 3.125 mg / Antihistamine
Safe for adults with
High Blood Pressure
Diabetes
√ Controls Cough
√ Relieves Sneezing
√ Relieves runny nose
SUGAR & ALCOHOL FREE
4.0 FL OZ (118mL)
SEGURO PARA ADULTOS CON PRESION ALTA Y DIABETES
Safetussin ®PM
Alivia la Tos
Durante la NocheDextromethorphan HBr 7.5 mg / Antiusivo
Doxylamine Succinate 3.125 mg / Antihistaminíco
Seguro para adultos con
Presión Alta
Diabetes
√ Calma la tos,
√ Alivia estornudos
√ Alivia goteo de la nariz
SINAZÚCAR Y SINALCOHOL
4 FL. OZ (118 mL)
Safetussin ®PM
NIGHT TIME
COUGH RELIEF
Cough Suppressant / Antihistamine
Up To
8 Hours of cough relief
to help you restSafetussin ®PM contains:
√ No decongestants
√ No acetaminophen
√ No sugar
√ No alcohol
√ No sorbitol
√ No fructose
√ No gluten
√ No dyes
Pharmacist Recommended Brand
Always read and follow label directions. Do not use longer than 7 consecutive days unless directed by a doctor. Ask a doctor if you have individual health questions.
Questions? Comments?
Call 1-800-824-4894
© 2023 Kramer Laboratories, Inc.
Bridgewater, NJ 08807 ● kramerlabs.com
K0723PACKAGE LABEL FOR 4 FL OZ (118 mL)
-
INGREDIENTS AND APPEARANCE
SAFETUSSIN PM NIGHTTIME COUGH RELIEF
dextromethorphan hbr, doxylamine succinate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55505-221 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN (UNII: 7355X3ROTS) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 7.5 mg in 5 mL DOXYLAMINE SUCCINATE (UNII: V9BI9B5YI2) (DOXYLAMINE - UNII:95QB77JKPL) DOXYLAMINE SUCCINATE 3.125 mg in 5 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GLYCERIN (UNII: PDC6A3C0OX) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) MINT (UNII: FV98Z8GITP) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55505-221-33 1 in 1 CARTON 07/28/2023 1 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC:55505-221-36 1 in 1 CARTON 07/28/2023 2 237 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 07/28/2023 Labeler - Kramer Laboratories (122720675)