Version 3.0.2
The following document provides guidelines for preparing the SPLIMAGE - an image file of an oral solid
dosage form that is submitted to the Food and Drug Administration (FDA) with SPL documents.
DOWNLOAD PDF
[ HTTPS / FTP ]
-
General Description
SPLIMAGE is for image files of oral solid dosage forms that are submitted to the Food and Drug Administration (FDA) with SPL documents. As part of an inter-agency collaboration with the FDA, the National Library of Medicine (NLM) studied the imaging of a broad range of oral solid dosage forms for pharmaceutical products. This study allowed the NLM to make the following technical recommendations for a SPLIMAGE file composition and format.
- Image should portray the visual characteristics of an oral solid dosage form such that one can readily identify distinguishing visual characteristics (with the exception of oral solid dosage form thickness).
- Image size is defined as 1024 pixels wide by 768 pixels high, square pixels, XGA size standard, and in 24-bit truecolor image, sRGB colorspace.
- Image coordinates are presented in standard (x,y) pixel positions where the origin position (0,0) is defined as being at the top left of the image. Frame areas are presented as (x1,y1,x2,y2) where the area of the frame spans from (x1,y1) to (x2,y2). See figure below for the coordinate system for the entire SPLIMAGE pixel space:
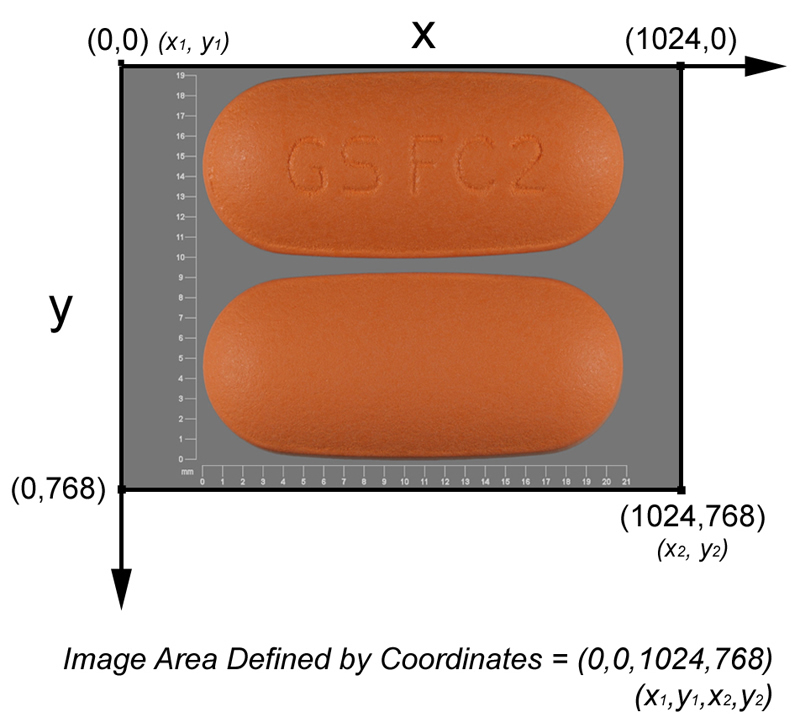
-
Oral solid dosage form images are composited in one of two possible arrangements based on the aspect ratio of the imaged oral solid dosage form:
- Vertical Oral Solid Dosage Form Layout [Section 3.1]
- Horizontal Oral Solid Dosage Form Layout [Section 3.2]
- Image file is required to be in JPEG format ISO/IEC 10918 with a file size limited to 150kB (kilobytes).
- Images should be included with SPL submissions as described in FDA guidance documents and specifications.
-
SPLIMAGE Characteristics
In order to capture the important visual characteristics of the oral solid dosage form, images submitted are required to include obverse (front) and reverse (back) views of the oral solid dosage form.
- Imprints, symbols, scores, and color patterns or variations should be in focus and clearly identifiable in the image submitted by the firm.
- Illumination artifacts (e.g., specular highlights, harsh shadows, etc.) should be limited. Exposure and white balance reference targets should be included in the imaging process to assure that illumination and color calibration is as accurate as possible. Size calibration targets should be included to assure that dimensional and scaling information is captured as accurately as possible.
- The oral solid dosage form views (obverse and reverse of the subject sample) should occupy the largest allowable area in the applicable layout window (Obverse View Window and Reverse View Window [defined in Sections 3.1.2 and 3.2.2]). Within the constraints of the applicable layout, the oral solid dosage form image should be orientated to allow for the most natural reading orientation for imprints and markings. Viewers should not normally need to rotate the submitted oral solid dosage form image in order to read it.
-
Product Image Layout Definition
Two layouts are defined: (i) Vertical Oral Solid Dosage Form Layout and (ii) Horizontal Oral Solid Dosage Form Layout. The appropriate layout definition is chosen based on the aspect ratio of the dosage form.
-
Vertical Oral Solid Dosage Form Layout Definition:
- Used for oral solid dosage form images with an aspect ratio ≥ 1.42. The oral solid dosage form image aspect ratio is defined as follows: longest oral solid dosage form image axis (horizontal or vertical) / shortest oral solid dosage form image axis (horizontal or vertical) - see example below:
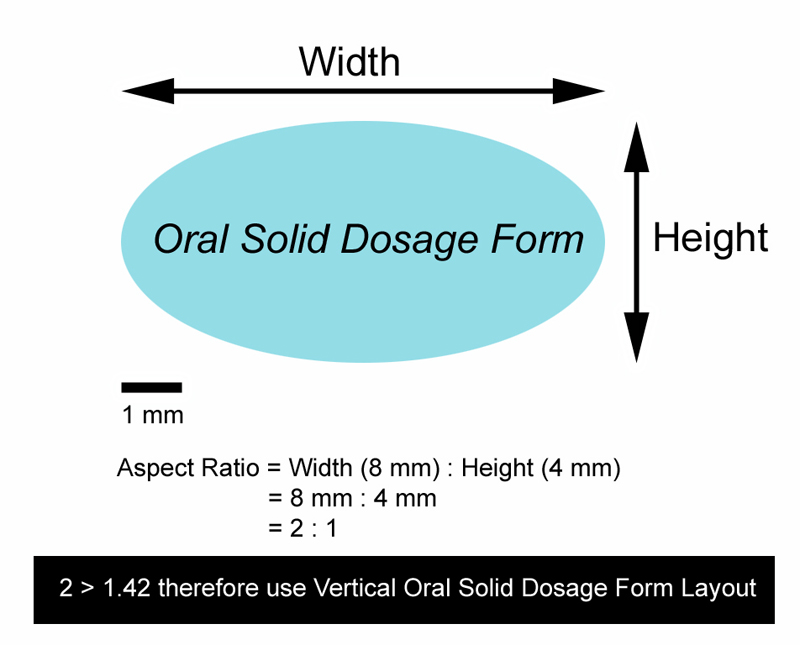
-
Vertical Oral Solid Dosage Form Image Layout Image Frame (1024x768) is divided into two primary windows (x1,y1,x2,y2):
- Obverse View Window = (52,8,1016,350)
- Reverse View Window = (52,374,1016,716)
-
Vertical Oral Solid Dosage Form Image Layout Safe Zones, which should be devoid of any sample view or scaling information include:
- Border Safe Zone = 8 pixels around the perimeter of Obverse and Reverse View windows.
- Inter-View Window Spacing Safe Zone = 32 pixel wide horizontal safe area, separating the Obverse View Window and Reverse View Window.
-
Oral Solid Dosage Form Image Sample Centering Coordinates (x,y):
- Obverse View Sample Center Coordinate (534,179)
- Reverse View Sample Center Coordinate (534,545)
- Vertical Oral Solid Dosage Formb Image Layout Coordinate Guide
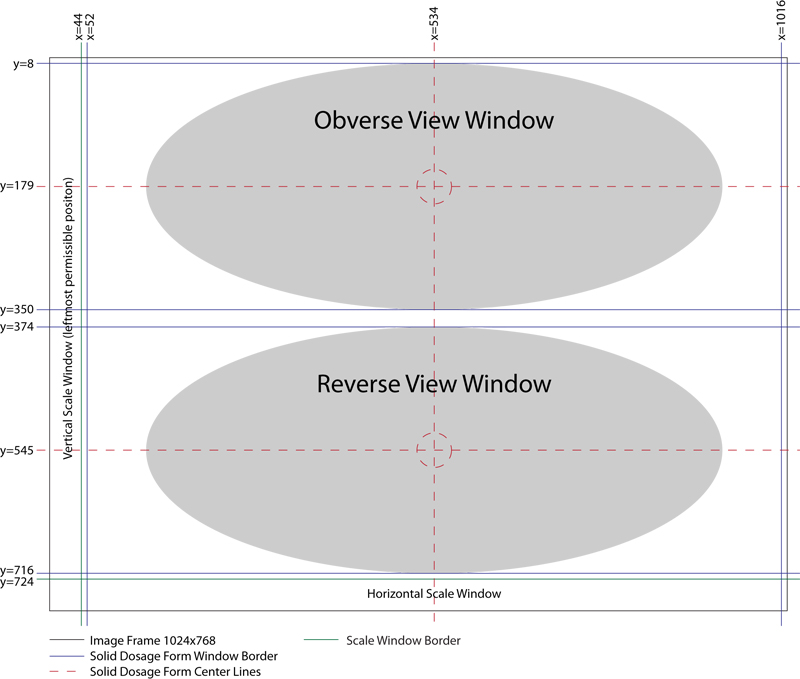
- Well Composed Vertical Oral Solid Dosage Form Image Layout Example

- Used for oral solid dosage form images with an aspect ratio ≥ 1.42. The oral solid dosage form image aspect ratio is defined as follows: longest oral solid dosage form image axis (horizontal or vertical) / shortest oral solid dosage form image axis (horizontal or vertical) - see example below:
-
Horizontal Oral Solid Dosage Form Image Layout Definition:
- Used for oral solid dosage form images with an aspect ratio < 1.42. The oral solid dosage form image aspect ratio is defined as follows: longest oral solid dosage form image axis (horizontal or vertical) / shortest oral solid dosage form image axis (horizontal or vertical) - see example below:
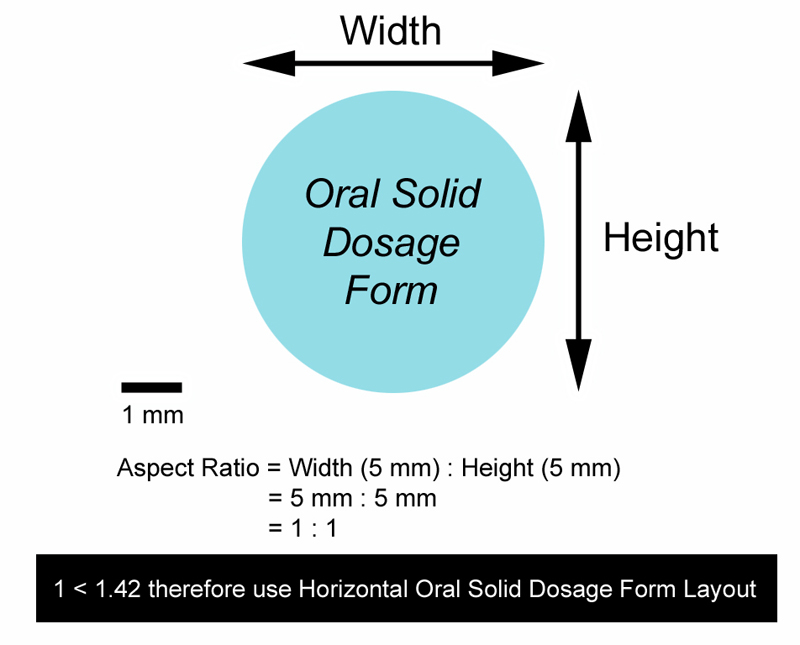
-
Horizontal Oral Solid Dosage Form Image Layout Image Frame (1024x768) is divided into two primary windows (x1,y1,x2,y2):
- Obverse View Window = (52,8,518,716)
- Reverse View Window = (550,8,1016,716)
-
Horizontal Oral Solid Dosage Form Image Layout Safe Zones, which should be devoid of any sample view or scaling information include:
- Border Safe Zone = 8 pixels around the perimeter of Obverse and Reverse View windows.
- Inter-View Window Spacing Safe Zone = 32 pixel wide vertical safe area, separating the Obverse View Window and Reverse View Window.
-
Oral Solid Dosage Form Image Sample Centering Coordinates (x,y):
- Obverse View Sample Center Coordinate (285,362)
- Reverse View Sample Center Coordinate (783,362)
- Horizontal Oral Solid Dosage Form Image Layout Coordinate Guide
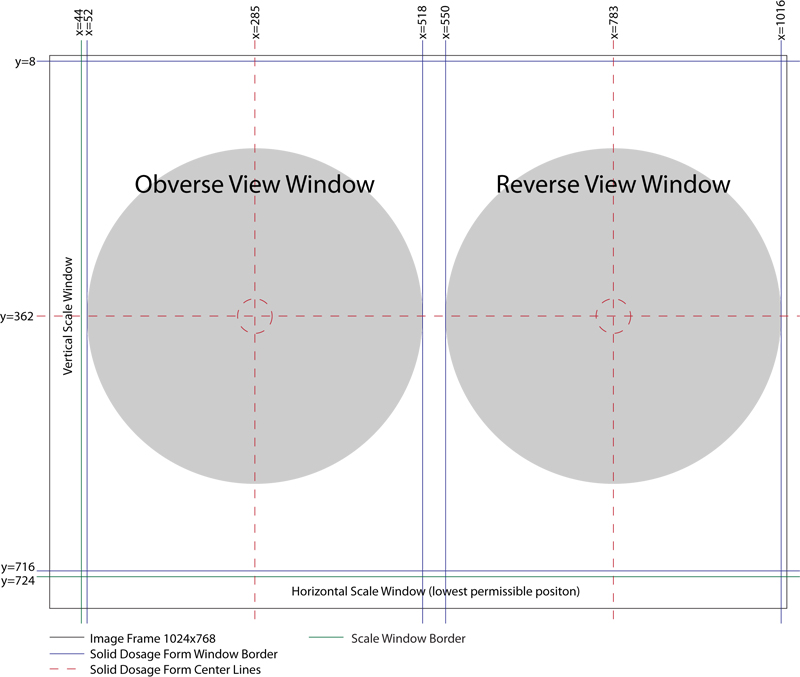
- Well Composed Horizontal Oral Solid Dosage Form Image Layout Example

- Used for oral solid dosage form images with an aspect ratio < 1.42. The oral solid dosage form image aspect ratio is defined as follows: longest oral solid dosage form image axis (horizontal or vertical) / shortest oral solid dosage form image axis (horizontal or vertical) - see example below:
-
Vertical Oral Solid Dosage Form Layout Definition:
-
Scale Windows
SPL document information contains oral solid dosage form size in millimeters (mm). Horizontal and vertical scales aid in the proper composition of the SPLIMAGE file and in the identification of oral solid dosage forms. Scales should be clearly marked in mm and be placed along both horizontal and vertical axes.
-
Vertical Layout Scale Windows:
- Vertical Layout - Vertical Scale Window Placement: place 8 pixels to the left of the left-most edge of the oral solid dosage form image. Leftmost permissible position (x1,y1,x2,y2): (0,0,44,768)
- Vertical Layout - Horizontal Scale Window (x1,y1,x2,y2): (0,724,1024,768)
-
Horizontal Layout Scale Windows:
- Horizontal Layout - Vertical Scale Window (x1,y1,x2,y2): (0,0,44,768)
- Horizontal Layout - Horizontal Scale Window: place 8 pixels below the bottom-most edge of the oral solid dosage form image. Lowest permissible position (x1,y1,x2,y2): (0,724,1024,768)
-
Vertical Layout Scale Windows:
-
Image Processing
- To eliminate shadows, background textures and unwanted imaging artifacts, oral solid dosage form images should be segmented from the photographic background used.
- Oral solid dosage form images must have a clean outline, placed against a medium gray (24-bit RGB value of 118,118,118) background.
- All image processing should be carried out to carefully ensure that proper scaling is applied at all times. Final SPLIMAGE files need to be correctly scaled and must preserve all original aspect ratios of the oral solid dosage forms being imaged.
- JPEG compression should be set to the highest quality offered by the creation software which will allow the SPLIMAGE file to be equal to or smaller than a 150kB file size limit.
-
SPLIMAGE Submission Instructions
- For information regarding submission of images to the FDA as part of a product SPL, please refer to the following website: https://www.fda.gov/industry/fda-resources-data-standards/structured-product-labeling-resources
-
Technical Support
For questions or comments on the SPLIMAGE file specification, please send an email to splimage@nlm.nih.gov. Questions regarding inclusion of the files in SPL drug listing submissions should be addressed to the FDA at spl@fda.hhs.gov.
Close
SPLIMAGE Specification Summary
| Image Size, & File Name |
|
| Image File Type & Compression |
|
| Image File Submission Instructions, Technical Assistance & SPLIMAGE Specification Feedback Instructions |
|
