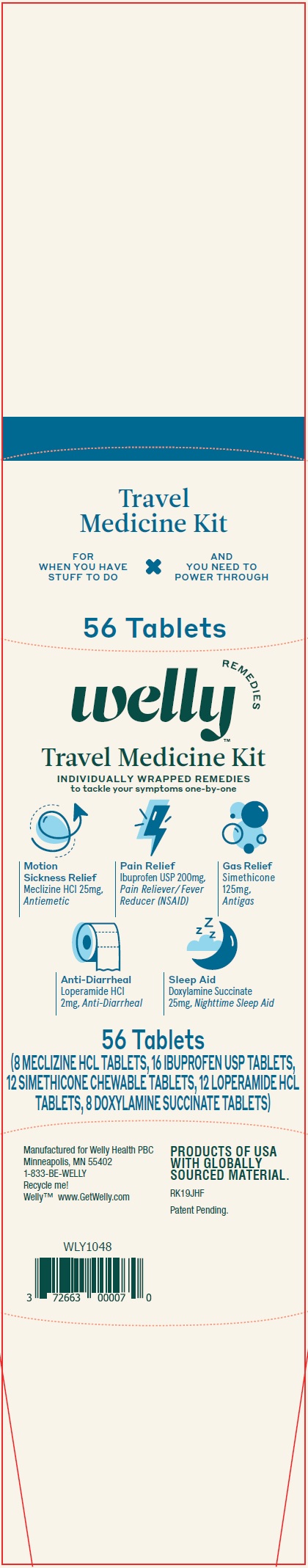
Label: WELLY TRAVEL MEDICINE KIT- meclizine hydrochloride, ibuprofen, loperamide hydrochloride, doxylamine succinate kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 72663-205-48, 72663-369-48, 72663-428-48, 72663-567-48, view more72663-632-48, 72663-746-48 - Packager: Welly Health PBC
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 15, 2020
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
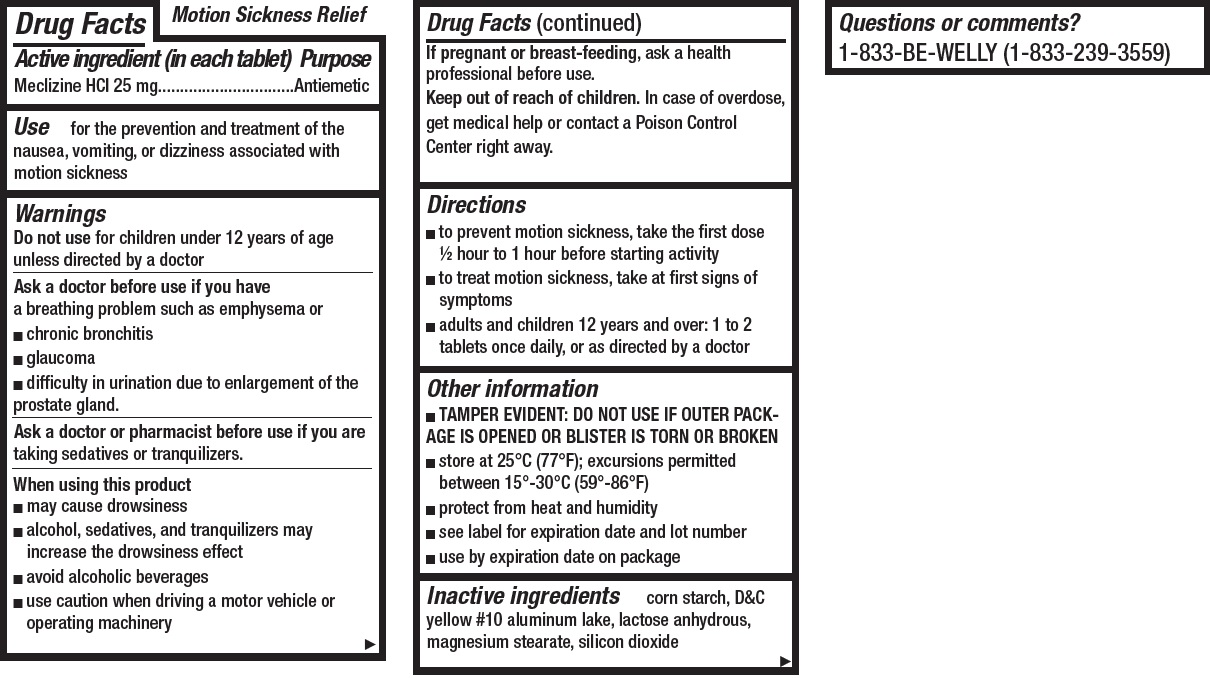
- Motion Sickness Relief, 8 tablets
- Drug Facts
- Active ingredient (in each tablet)
- Use
-
Warnings
Ask a doctor before use if you have
a breathing problem such as emphysema or
- chronic bronchitis
- glaucoma
- difficulty in urination due to enlargement of the prostate gland.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
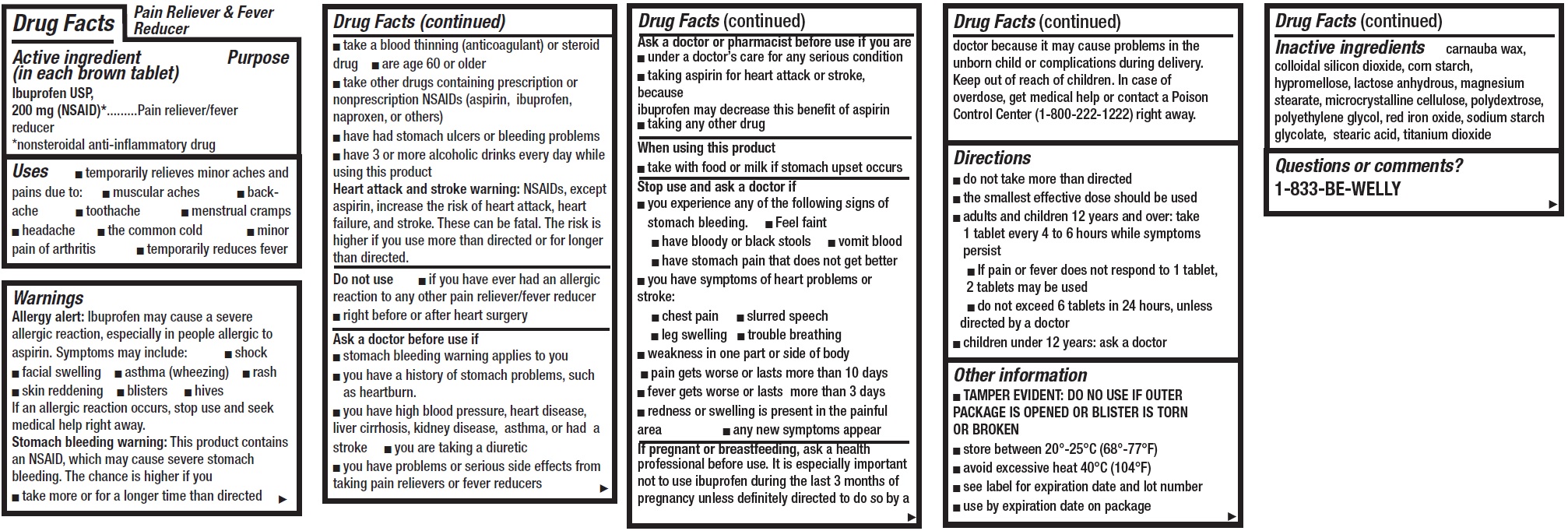
- Pain Relief and Fever Reducer, 16 tablets
- Drug Facts
- Active ingredient (in each brown tablet)
- Uses
-
Warnings
Allergy alert: Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include:
- shock
- facial swelling
- asthma (wheezing)
- rash
- skin reddening
- blisters
- hives
If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you
- take more or for a longer time than directed
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
- have had stomach ulcers or bleeding problems
- have 3 or more alcoholic drinks every day while using this product
Heart attack and stroke warning: NSAIDs, except aspirin, increase the risk of heart attack, heart failure, and stroke. These can be fatal. The risk is higher if you use more than directed or for longer than directed.
Do not use
- if you have ever had an allergic reaction to any other pain reliever/fever reducer
- right before or after heart surgery
Ask a doctor before use if
- stomach bleeding warning applies to you
- you have a history of stomach problems, such as heartburn.
- you have high blood pressure, heart disease, liver cirrhosis, kidney disease, asthma, or had a stroke
- you are taking a diuretic
- you have problems or serious side effects from taking pain relievers or fever reducers
Ask a doctor or pharmacist before use if you are
- under a doctor’s care for any serious condition
- taking aspirin for heart attack or stroke, because uprofen may decrease this benefit of aspirin
- taking any other drug
Stop use and ask a doctor if
- you experience any of the following signs of stomach bleeding.
- Feel faint
- have bloody or black stools
- vomit blood
- have stomach pain that does not get better
- you have symptoms of heart problems or stroke:
- chest pain
- slurred speech
- leg swelling
- trouble breathing
- weakness in one part or side of body
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present in the painful area
- any new symptoms appear
-
Directions
- do not take more than directed
- the smallest effective dose should be used
- adults and children 12 years and over: take 1 tablet every 4 to 6 hours while symptoms persist
- If pain or fever does not respond to 1 tablet, 2 tablets may be used
- do not exceed 6 tablets in 24 hours, unless directed by a doctor
- children under 12 years: ask a doctor
- Other information
- Inactive ingredients
- Questions or comments?
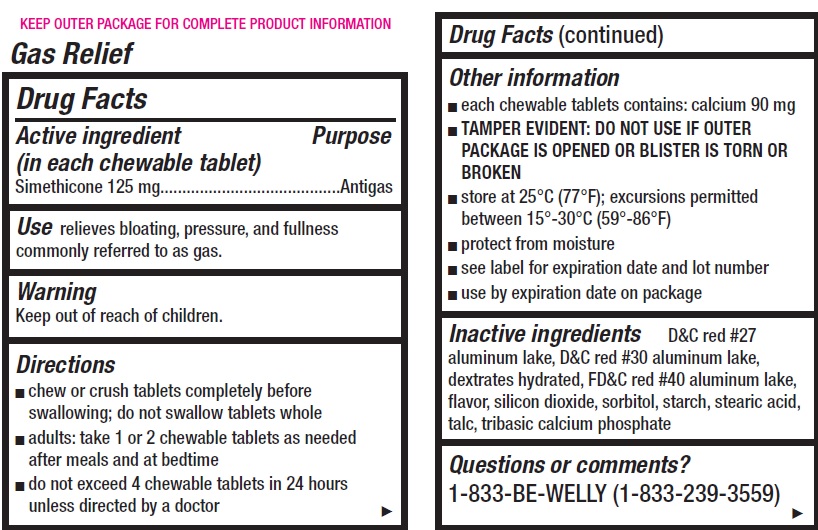
- Gas Relief, 12 chewable tablets
- Drug Facts
- Active ingredient (in each chewable tablet)
- Use
- Warning
- Directions
-
Other information
- each chewable tablets contains: calcium 90 mg
- TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
- protect from moisture
- see label for expiration date and lot number
- use by expiration date on package
- Inactive ingredients
- Questions or comments?
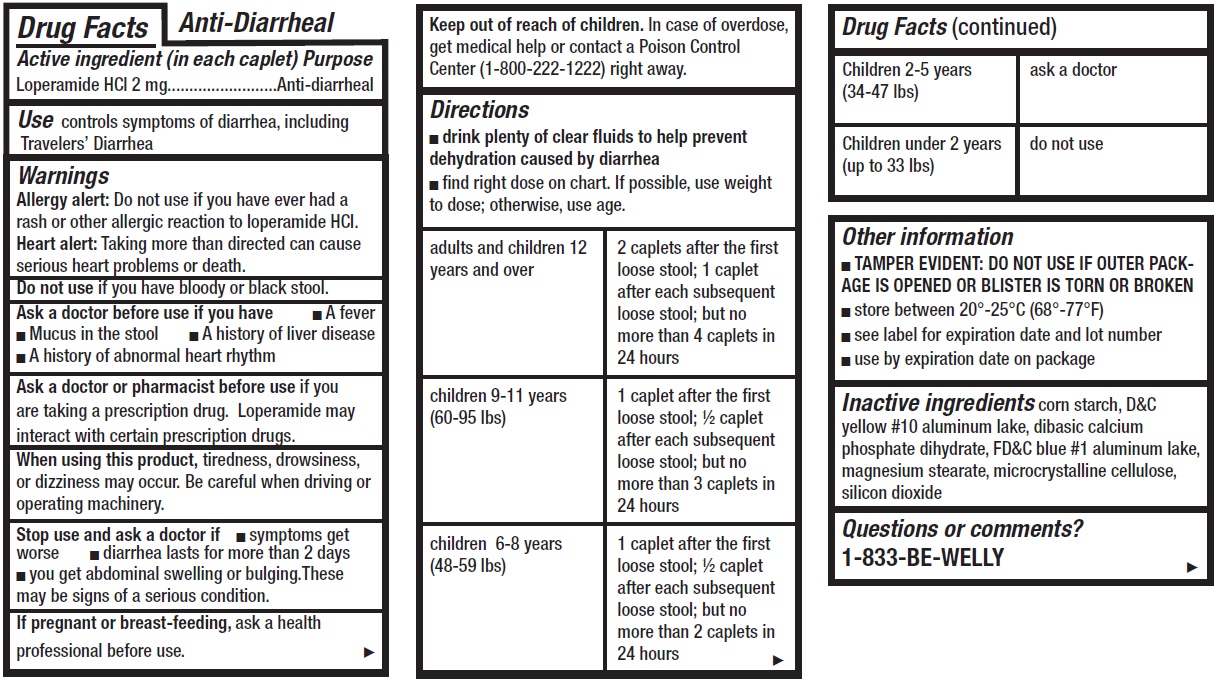
- Anti-Diarrheal, 12 tablets
- Drug Facts
- Active ingredient (in each caplet)
- Use
-
Warnings
Allergy alert: Do not use if you have ever had a rash or other allergic reaction to loperamide HCI.
Heart alert: Taking more than directed can cause serious heart problems or death.Ask a doctor before use if you have
- A fever
- Mucus in the stool
- A history of liver disease
- A history of abnormal heart rhythm
Ask a doctor or pharmacist before use
if you are taking a prescription drug. Loperamide may interact with certain prescription drugs.
When using this product,
tiredness, drowsiness, or dizziness may occur. Be careful when driving or operating machinery.
-
Directions
- drink plenty of clear fluids to help prevent dehydration caused by diarrhea
- find right dose on chart. If possible, use weight to dose; otherwise, use age.
adults and children 12 years and over 2 caplets after the first loose stool; 1 caplet after each subsequent loose stool; but no more than 4 caplets in 24 hours children 9-11 years (60-95 lbs) 1 caplet after the first loose stool; ½ caplet after each subsequent loose stool; but no more than 3 caplets in 24 hours children 6-8 years (48-59 lbs) 1 caplet after the first loose stool; ½ caplet after each subsequent loose stool; but no more than 2 caplets in 24 hours Children 2-5 years (34-47 lbs) ask a doctor Children under 2 years (up to 33 lbs) do not use - Other information
- Inactive ingredients
- Questions or comments?
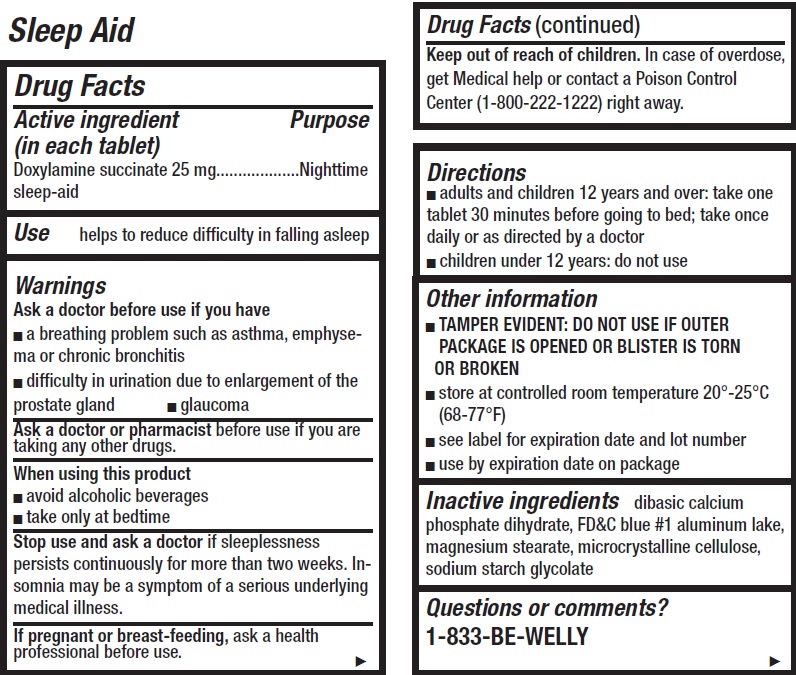
- Sleep Aid, 8 tablets
- Drug Facts
- Active ingredient (in each tablet)
- Use
-
Warnings
Ask a doctor before use if you have
- a breathing problem such as asthma, emphysema or chronic bronchitis
- difficulty in urination due to enlargement of the prostate gland
- glaucoma
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- Package Labeling:(72663-205-48)
- undefined
- Package Labeling:(72663-428-48)
- Package Labeling:(72663-746-48)
- Package Labeling:(72663-567-48)
- Package Labeling:(72663-369-48)
-
INGREDIENTS AND APPEARANCE
WELLY TRAVEL MEDICINE KIT
meclizine hydrochloride, ibuprofen, loperamide hydrochloride, doxylamine succinate kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72663-205 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72663-205-48 1 in 1 KIT 04/06/2020 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BLISTER PACK 8 Part 2 1 BOTTLE 16 Part 3 2 BLISTER PACK 12 Part 4 1 BLISTER PACK 12 Part 5 1 BLISTER PACK 8 Part 1 of 5 MOTION SICKNESS RELIEF
meclizine hydrochloride tabletProduct Information Item Code (Source) NDC:72663-632 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MECLIZINE HYDROCHLORIDE (UNII: HDP7W44CIO) (MECLIZINE - UNII:3L5TQ84570) MECLIZINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) D&C YELLOW NO. 10 ALUMINUM LAKE (UNII: CQ3XH3DET6) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) MAGNESIUM STEARATE (UNII: 70097M6I30) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color yellow Score no score Shape ROUND Size 9mm Flavor Imprint Code 44403 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72663-632-48 8 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part336 04/06/2020 Part 2 of 5 PAIN RELIEF AND FEVER REDUCER
ibuprofen tabletProduct Information Item Code (Source) NDC:72663-428 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IBUPROFEN (UNII: WK2XYI10QM) (IBUPROFEN - UNII:WK2XYI10QM) IBUPROFEN 200 mg Inactive Ingredients Ingredient Name Strength CARNAUBA WAX (UNII: R12CBM0EIZ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STARCH, CORN (UNII: O8232NY3SJ) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYDEXTROSE (UNII: VH2XOU12IE) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) FERRIC OXIDE RED (UNII: 1K09F3G675) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color brown Score no score Shape ROUND Size 10mm Flavor Imprint Code 44291 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72663-428-48 16 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 04/06/2020 Part 3 of 5 GAS RELIEF
dimethicone tablet, chewableProduct Information Item Code (Source) NDC:72663-746 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 125 mg Inactive Ingredients Ingredient Name Strength D&C RED NO. 27 ALUMINUM LAKE (UNII: ZK64F7XSTX) D&C RED NO. 30 (UNII: 2S42T2808B) FD&C RED NO. 40 (UNII: WZB9127XOA) METHYL SALICYLATE (UNII: LAV5U5022Y) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SORBITOL (UNII: 506T60A25R) STEARIC ACID (UNII: 4ELV7Z65AP) TALC (UNII: 7SEV7J4R1U) TRIBASIC CALCIUM PHOSPHATE (UNII: 91D9GV0Z28) Product Characteristics Color pink Score no score Shape ROUND Size 14mm Flavor Imprint Code 44608 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72663-746-48 2 in 1 KIT 1 6 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part332 04/06/2020 Part 4 of 5 ANTI DIARRHEAL
loperamide hydrochloride tabletProduct Information Item Code (Source) NDC:72663-567 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LOPERAMIDE HYDROCHLORIDE (UNII: 77TI35393C) (LOPERAMIDE - UNII:6X9OC3H4II) LOPERAMIDE HYDROCHLORIDE 2 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) D&C YELLOW NO. 10 ALUMINUM LAKE (UNII: CQ3XH3DET6) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) FD&C BLUE NO. 1 ALUMINUM LAKE (UNII: J9EQA3S2JM) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color green (Light) Score 2 pieces Shape OVAL Size 10mm Flavor Imprint Code 44375 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72663-567-48 12 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076497 04/06/2020 Part 5 of 5 SLEEP AID
doxylamine succinate tabletProduct Information Item Code (Source) NDC:72663-369 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXYLAMINE SUCCINATE (UNII: V9BI9B5YI2) (DOXYLAMINE - UNII:95QB77JKPL) DOXYLAMINE SUCCINATE 25 mg Inactive Ingredients Ingredient Name Strength DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) FD&C BLUE NO. 1 ALUMINUM LAKE (UNII: J9EQA3S2JM) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Product Characteristics Color blue Score 2 pieces Shape OVAL Size 10mm Flavor Imprint Code 44386 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72663-369-48 8 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 04/06/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part336 04/06/2020 Labeler - Welly Health PBC (116766884)