Label: GUAIFENESIN AND PSEUDOEPHEDRINE HCL tablet, extended release
- NDC Code(s): 46122-801-08
- Packager: Amerisource Bergen
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 3, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purposes
-
Uses
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
- temporarily relieves nasal congestion due to
- common cold
- hay fever
- upper respiratory allergies
- temporarily restores freer breathing through the nose
- promotes nasal and/or sinus drainage
- temporarily relieves sinus congestion and pressure
-
Warnings
- Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- Ask a doctor before use if you have
- When using this product
- Stop use and ask a doctor if
- If pregnant or breast-feeding,
- Keep out of reach of children.
-
Directions
- do not crush, chew, or break extended-release tablet
- take with a full glass of water
- this product can be administered without regard for timing of meals
- adults and children 12 years and older: 2 extended-release tablets every 12 hours; not more than 4 extended-release tablets in 24 hours
- children under 12 years of age: do not use
- Other information
- Inactive ingredients
- Questions?
-
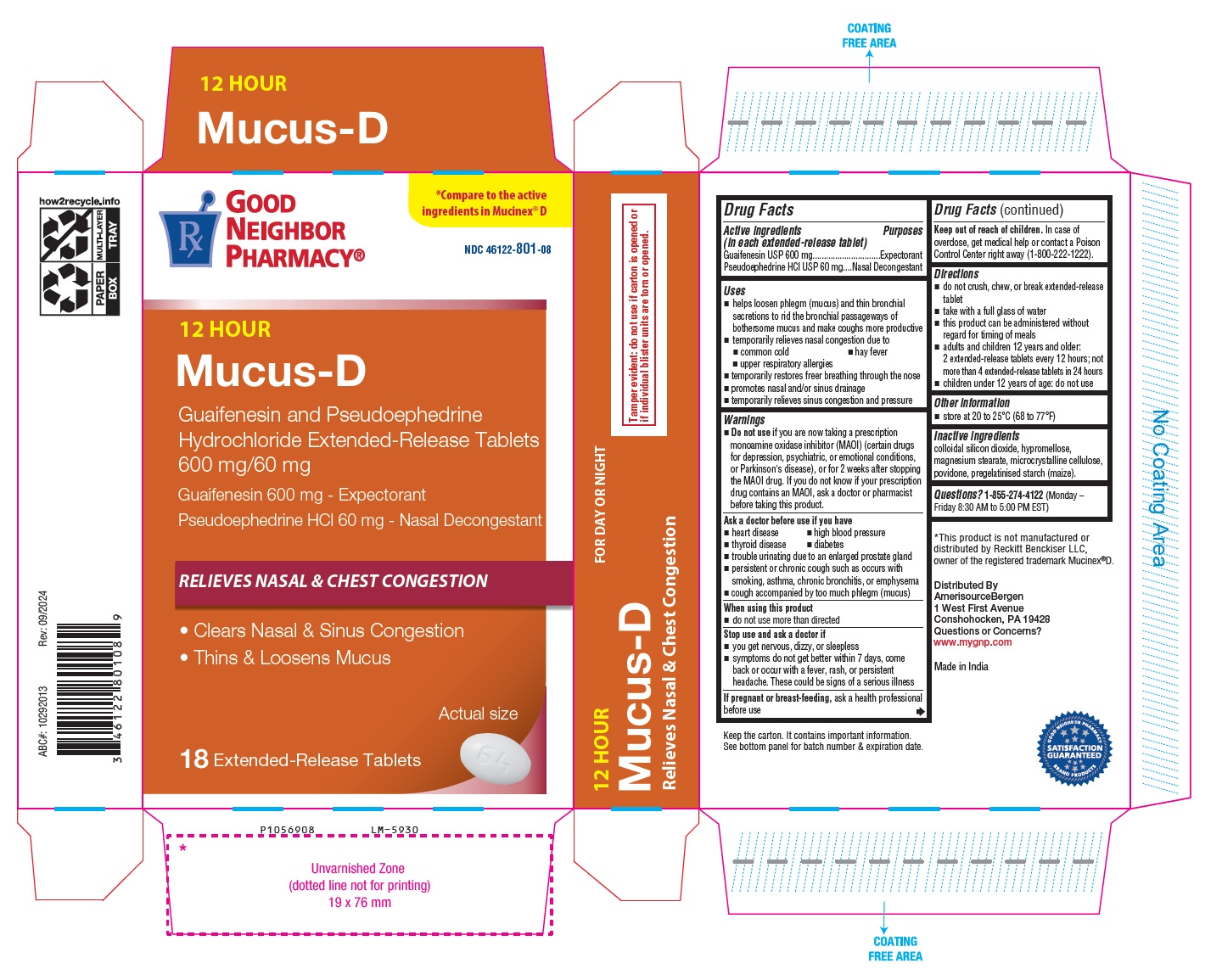
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 600 mg/60 mg (18 Tablet Carton Label)
℞ GOOD *Compare to the
NEIGHBOR active ingredients in Mucinex® D
PHARMACY®
NDC 46122-801-08
12 HOUR
Mucus-D
Guaifenesin and Pseudoephedrine
Hydrochloride Extended-Release Tablets
600 mg/60 mg
Guaifenesin 600 mg - Expectorant
Pseudoephedrine HCl 60 mg - Nasal Decongestant
RELIEVES NASAL & CHEST CONGESTION
• Clears Nasal & Sinus Congestion
• Thins & Loosens Mucus
Actual size
18 Extended-Release Tablets
-
INGREDIENTS AND APPEARANCE
GUAIFENESIN AND PSEUDOEPHEDRINE HCL
guaifenesin and pseudoephedrine hcl tablet, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:46122-801 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 600 mg PSEUDOEPHEDRINE HYDROCHLORIDE (UNII: 6V9V2RYJ8N) (PSEUDOEPHEDRINE - UNII:7CUC9DDI9F) PSEUDOEPHEDRINE HYDROCHLORIDE 60 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYPROMELLOSE 2208 (15000 MPA.S) (UNII: Z78RG6M2N2) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE 101 (UNII: 7T9FYH5QMK) MICROCRYSTALLINE CELLULOSE 102 (UNII: PNR0YF693Y) POVIDONE K25 (UNII: K0KQV10C35) POVIDONE K90 (UNII: RDH86HJV5Z) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color WHITE (white to off-white) Score no score Shape OVAL (biconvex) Size 14mm Flavor Imprint Code 64;X Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:46122-801-08 1 in 1 CARTON 10/01/2024 1 18 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA213203 10/01/2024 Labeler - Amerisource Bergen (007914906) Registrant - Aurobindo Pharma Limited (650082092) Establishment Name Address ID/FEI Business Operations APL HEALTHCARE LIMITED 650918514 ANALYSIS(46122-801) , MANUFACTURE(46122-801)

