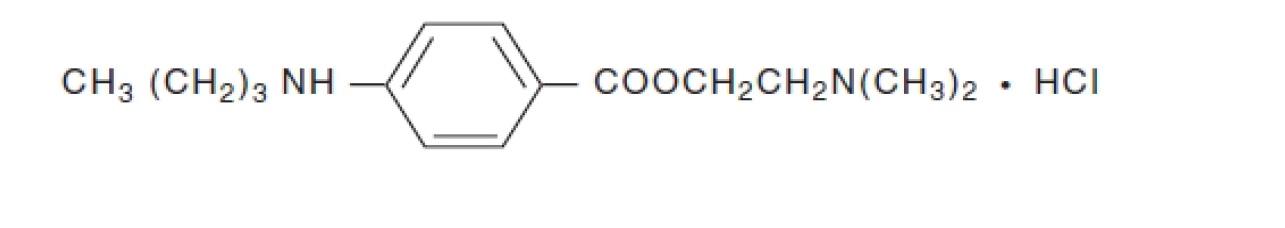Label: TETRACAINE HYDROCHLORIDE solution/ drops
- NDC Code(s): 0187-0920-05
- Packager: Bausch Health US LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated April 6, 2020
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Tetracaine Hydrochloride Ophthalmic Solution, USP 0.5% safely and effectively. See full prescribing information for Tetracaine ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE Tetracaine Hydrochloride Ophthalmic Solution, USP 0.5% is indicated for procedures requiring a rapid and short-acting topical ophthalmic anesthetic.
-
2 DOSAGE AND ADMINISTRATION One drop topically in the eye(s) as needed.
-
3 DOSAGE FORMS AND STRENGTHS Tetracaine Hydrochloride Ophthalmic Solution, USP 0.5% is a clear, colorless, ophthalmic solution containing 0.5% w/v tetracaine hydrochloride equivalent to tetracaine 0.44% w/v.
-
4 CONTRAINDICATIONS (What is this?)Tetracaine Hydrochloride Ophthalmic Solution, USP 0.5% should not be used in patients with a history of hypersensitivity to any component of this preparation.
-
5 WARNINGS AND PRECAUTIONS 5.1 Corneal Injury with Intracameral Use - Not for injection or intraocular use. Do not use intracamerally because use of Tetracaine Hydrochloride Ophthalmic Solution, USP 0.5% may lead to ...
-
6 ADVERSE REACTIONS The following serious ocular adverse reactions are described elsewhere in the labeling: • Corneal Injury with Intracameral Use [See Warnings and Precautions (5.1)] • Corneal Toxicity [See ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - There are no adequate and well-controlled studies with Tetracaine Hydrochloride Ophthalmic Solution, USP 0.5% in pregnant women. Animal developmental and ...
-
10 OVERDOSAGE Prolonged use of a topical ocular anesthetic including Tetracaine Hydrochloride Ophthalmic Solution, USP 0.5% may produce permanent corneal opacification and ulceration with accompanying visual ...
-
11 DESCRIPTION Tetracaine Hydrochloride Ophthalmic Solution, USP 0.5% is a sterile, clear, colorless, topical local anesthetic for ophthalmic use only containing tetracaine hydrochloride as the active ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Tetracaine blocks sodium ion channels required for the initiation and conduction of neuronal impulses thereby affecting local anesthesia. 12.3 Pharmacokinetics ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Studies to assess the genotoxicity of tetracaine hydrochloride have not been reported in the published literature. Long-term animal ...
-
14 CLINICAL STUDIES Topical administration of Tetracaine Hydrochloride Ophthalmic Solution, USP 0.5% results in localized temporary anesthesia. The maximum effect is achieved within 10–20 seconds after instillation ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING Tetracaine Hydrochloride Ophthalmic Solution, USP 0.5% is supplied as a sterile, aqueous, topical ophthalmic solution in a low-density polyethylene plastic dropper bottle with a low-density ...
-
17 PATIENT COUNSELING INFORMATION Eye Care Precaution - Do not touch the dropper tip to any surface as this may contaminate the solution. Advise patients that, due to the effect of the anesthetic, their eyes will be insensitive ...
-
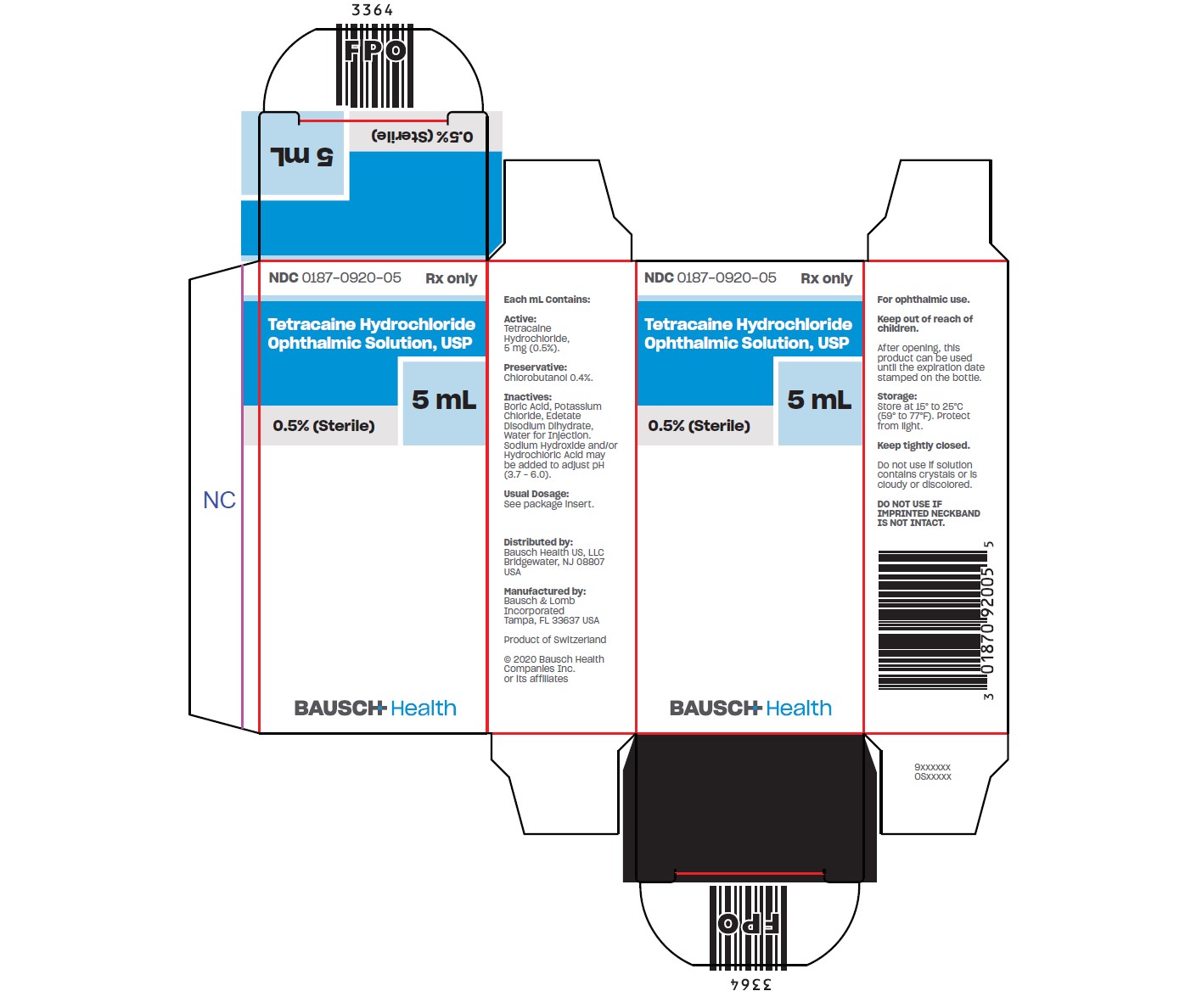
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL- Carton 5 mL
(What is this?)NDC 0187-0920-05 - Rx only - Tetracaine - Hydrochloride - Ophthalmic - Solution, USP - 0.05% (Sterile) 5 mL - BAUSCH Health
-
INGREDIENTS AND APPEARANCEProduct Information