Label: 4189 FIRST AID KIT kit
- NDC Code(s): 0498-0100-01, 0498-0143-04, 0498-0903-34, 0498-4189-01
- Packager: Honeywell Safety Products USA, INC
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated May 30, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
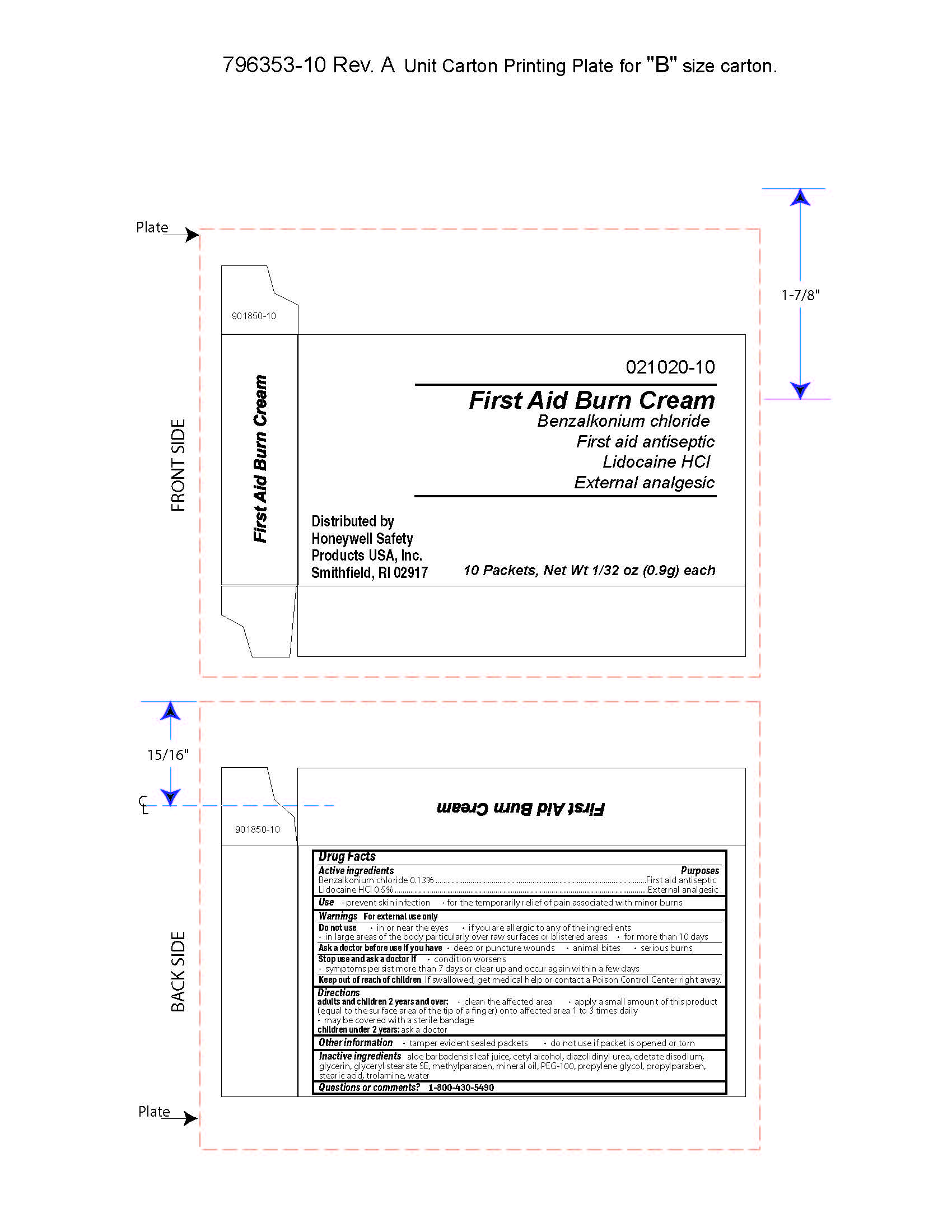
- First Aid Burn Cream Active ingredient
- First Aid Burn Cream Purpose
- First Aid Burn Cream Uses
- First Aid Burn Cream Warnings
- First Aid Burn Cream Directions
- First Aid Burn Cream Other information
- First Aid Burn Cream Inactive ingredients
- First Aid Burn Cream Questions
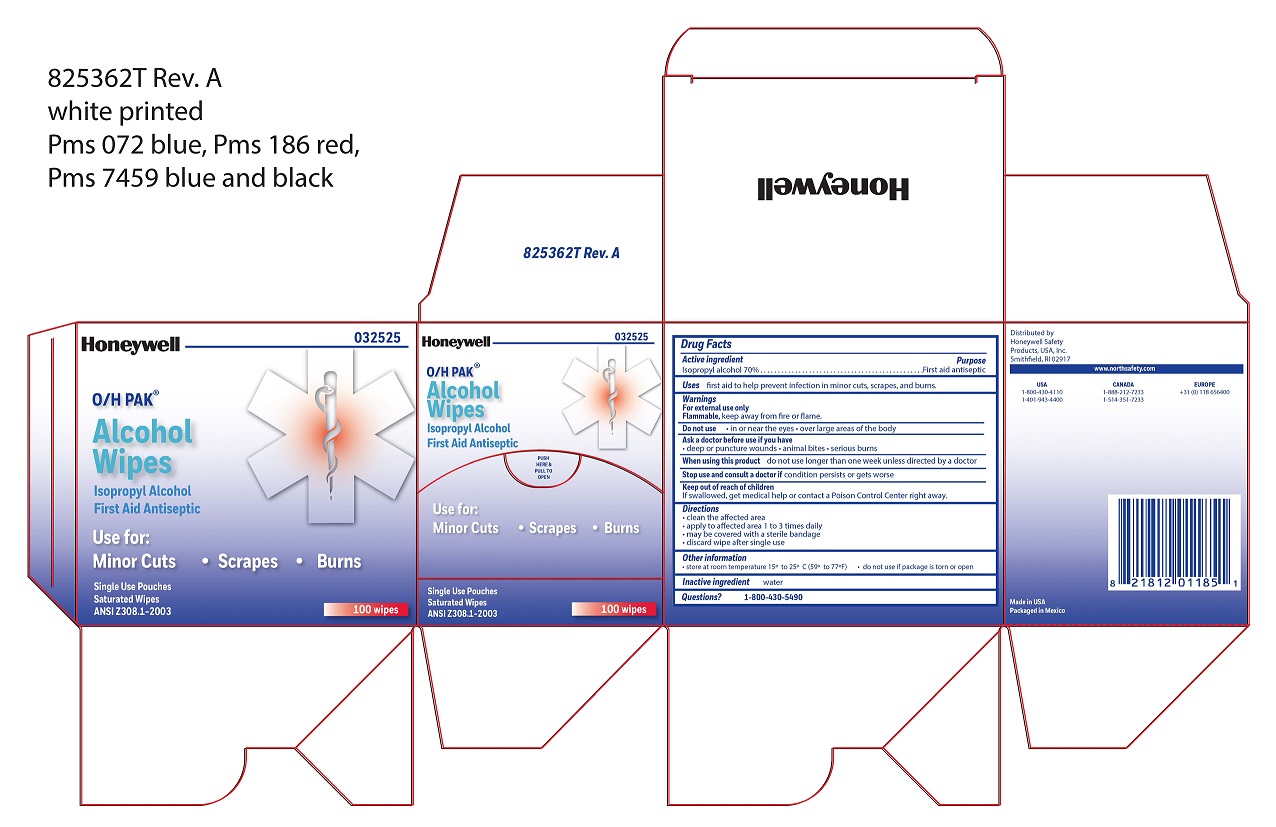
- Alcohol Wipe Active ingredient
- Alcohol Wipe Purpose
- Alcohol Wipe Uses
- Alcohol Wipe Warnings
- Alcohol Wipe Directions
- Alcohol Wipe Other information
- Alcohol Wipe Inactive ingredient
- Alcohol Wipe Questions
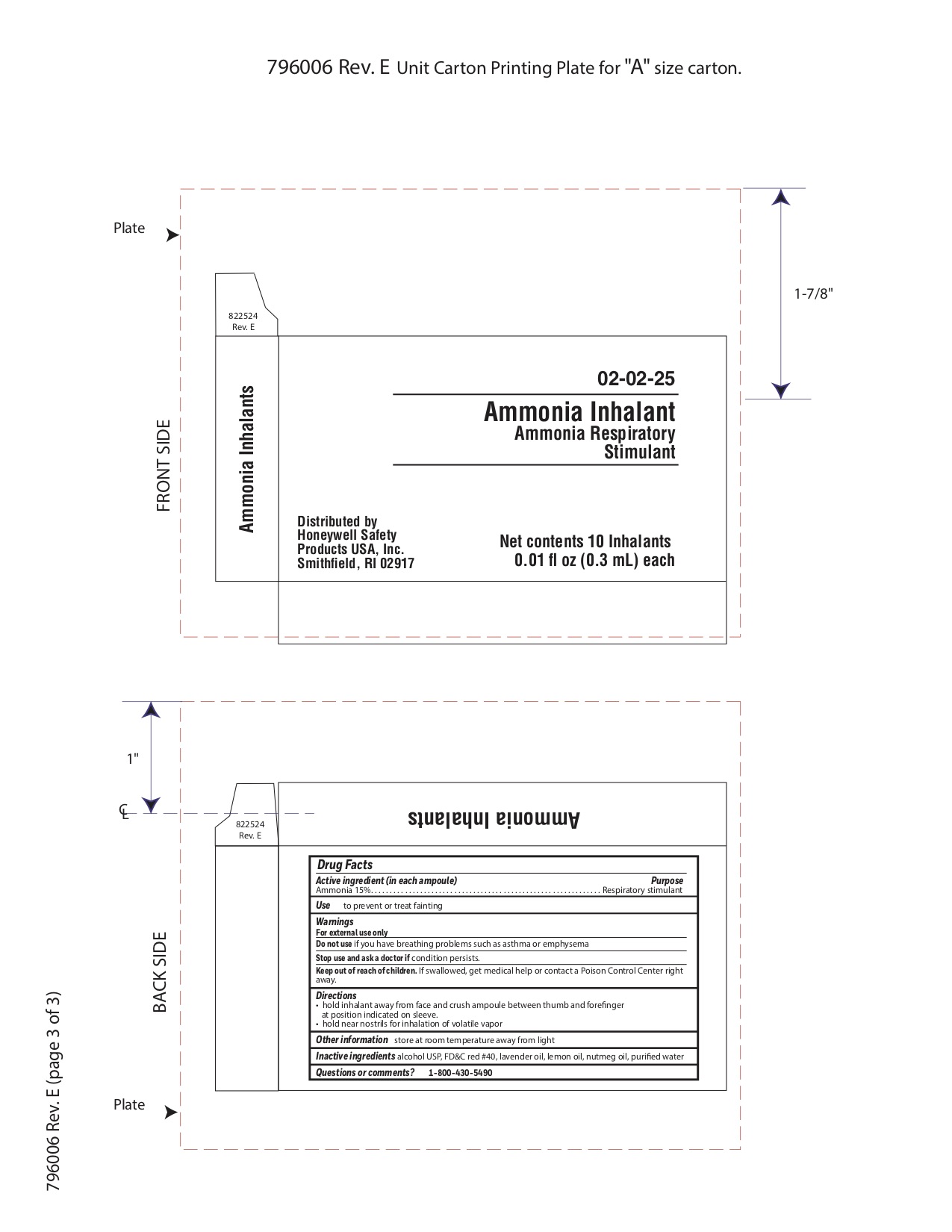
- Ammonia Inhalent Active ingredient (in each ampule)
- Ammonia Inhalent Purpose
- Ammonia Inhalent Uses
- Ammonia Inhalent Warnings
- Ammonia Inhalent Directions
- Ammonia Inhalent Other information
- Ammonia Inhalent Inactive ingredients
- Ammonia Inhalent Questions
- Eyewash Active ingredient
- Eyewash Purpose
- Eyewash Uses
-
Eyewash
Warnings
For external use only Obtain immediate medical treatment for all open wounds in or near eyes. To avoid contamination, do not touch tip of container to any surface. Do not reuse. Once opened, discard.
Do not use
- if solution changes color or becomes cloudy
- if you have open wounds in or near the eyes, get medical help right away.
- Eyewash Directions
- Eyewash Inactive ingredients
- Eyewash Questions
-
4189
010742-3236 Kit Contents
1 FIRST AID BURN CREAM 6 PER
2 AMMONIA INHALANTS 10 PER
1 TRIANGULAR BDG, NON-STERILE
2 GAUZE PADS, 3" X 3", 4 PER
1 FORCEPS & SCISSORS, 1 EA
1 GAUZE BANDAGE, 2" X 6 YD,2 PER
1 BUFFERED EYE WASH 1 OZ BTL
4 ADH BDG,PLSTIC,3/4"X3",16 PER
1 ALCOHOL PREP PADS 10P
1 ADHESIVE TAPE W/P 1/2"X 5 YD
LBL STOCK 6-3/8"X4"
1 LBL STOCK 3"x1-7/8"
1 KIT STL 16 UN (VERTICAL)
1 LABL INSTR FA REV A
- First Aid Burn Cream Principal Display Panel
- Alcohol Wipe Principal Display Panel
- Ammonia Inahalent Principal Display Panel
- Eyewash Principal Display Panel
- 4189 Kit Label 010742-3236
-
INGREDIENTS AND APPEARANCE
4189 FIRST AID KIT
4189 first aid kit kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0498-4189 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-4189-01 1 in 1 KIT; Type 0: Not a Combination Product 10/18/2018 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 6 PACKET 5.4 g Part 2 1 BOTTLE 30 mL Part 3 10 POUCH 4 mL Part 1 of 3 FIRST AID BURN
benzalkonium chloride, lidocaine hydrochloride creamProduct Information Item Code (Source) NDC:0498-0903 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 g LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE 0.5 g in 100 g Inactive Ingredients Ingredient Name Strength PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ALOE VERA LEAF (UNII: ZY81Z83H0X) WATER (UNII: 059QF0KO0R) STEARIC ACID (UNII: 4ELV7Z65AP) METHYLPARABEN (UNII: A2I8C7HI9T) CETYL ALCOHOL (UNII: 936JST6JCN) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) LIGHT MINERAL OIL (UNII: N6K5787QVP) EDETATE DISODIUM (UNII: 7FLD91C86K) TROLAMINE (UNII: 9O3K93S3TK) GLYCERIN (UNII: PDC6A3C0OX) PROPYLPARABEN (UNII: Z8IX2SC1OH) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-0903-34 10 in 1 BOTTLE, UNIT-DOSE 1 0.9 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 12/20/2017 Part 2 of 3 EYESALINE EMERGENCY EYEWASH
purified water liquidProduct Information Item Code (Source) NDC:0498-0100 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 98.6 mL in 100 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-0100-01 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 12/18/2018 Part 3 of 3 ALCOHOL WIPE
isopropyl alcohol swabProduct Information Item Code (Source) NDC:0498-0143 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-0143-04 0.4 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/18/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/18/2018 08/30/2025 Labeler - Honeywell Safety Products USA, INC (118768815)