Label: GUAIFENESIN AND PSEUDOEPHEDRINE HCL tablet, extended release
- NDC Code(s): 30142-137-12, 30142-137-17
- Packager: KROGER COMPANY
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 1, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purposes
-
Uses
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
- temporarily relieves nasal congestion due to:
- common cold
- hay fever
- upper respiratory allergies
- temporarily restores freer breathing through the nose
- promotes nasal and/or sinus drainage
- temporarily relieves sinus congestion and pressure
-
Warnings
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- Ask a doctor before use if you have
- When using this product
- Stop use and ask a doctor if
- If pregnant or breast-feeding,
- Keep out of reach of children.
- Directions
- Other information
- Inactive ingredients
- Questions?
-
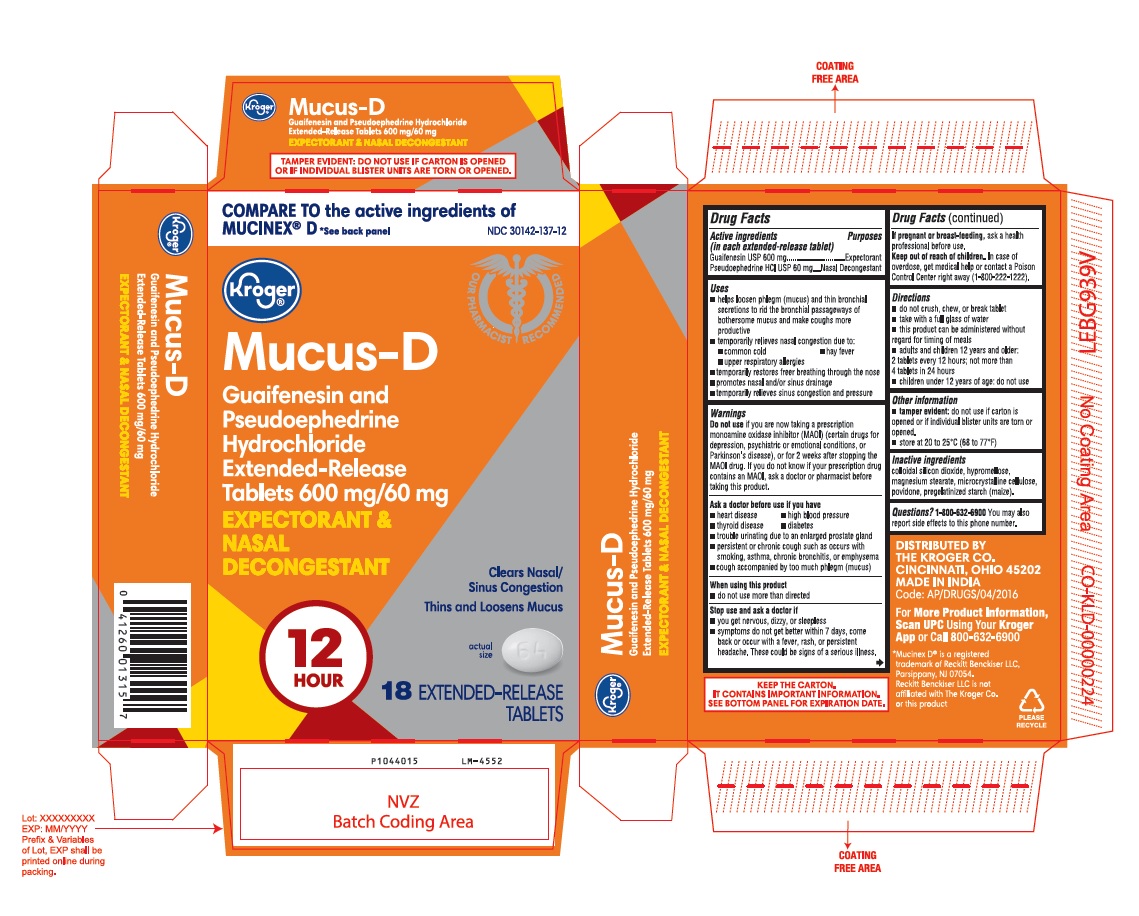
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 600 mg/60 mg (18 Tablet Carton Label)
COMPARE TO the active ingredients of
MUCINEX® D *See back panel NDC 30142-137-12
Kroger®
Mucus-D
Guaifenesin and
Pseudoephedrine
Hydrochloride
Extended-Release
Tablets 600 mg/60 mg
EXPECTORANT &
NASAL
DECONGESTANT
12
HOUR
Clears Nasal/
Sinus Congestion
Thins and Loosens Mucus
actual
size
18 EXTENDED-RELEASE
TABLETS
-
INGREDIENTS AND APPEARANCE
GUAIFENESIN AND PSEUDOEPHEDRINE HCL
guaifenesin and pseudoephedrine hcl tablet, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:30142-137 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 600 mg PSEUDOEPHEDRINE HYDROCHLORIDE (UNII: 6V9V2RYJ8N) (PSEUDOEPHEDRINE - UNII:7CUC9DDI9F) PSEUDOEPHEDRINE HYDROCHLORIDE 60 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYPROMELLOSE 2208 (15000 MPA.S) (UNII: Z78RG6M2N2) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE 101 (UNII: 7T9FYH5QMK) MICROCRYSTALLINE CELLULOSE 102 (UNII: PNR0YF693Y) POVIDONE K25 (UNII: K0KQV10C35) POVIDONE K90 (UNII: RDH86HJV5Z) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color WHITE (white to off-white) Score no score Shape OVAL (biconvex) Size 14mm Flavor Imprint Code 64;X Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:30142-137-12 1 in 1 CARTON 04/16/2021 1 18 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:30142-137-17 2 in 1 CARTON 04/16/2021 2 18 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA213203 04/16/2021 Labeler - KROGER COMPANY (006999528) Registrant - Aurohealth LLC (078728447) Establishment Name Address ID/FEI Business Operations APL HEALTHCARE LIMITED 650918514 ANALYSIS(30142-137) , MANUFACTURE(30142-137)

