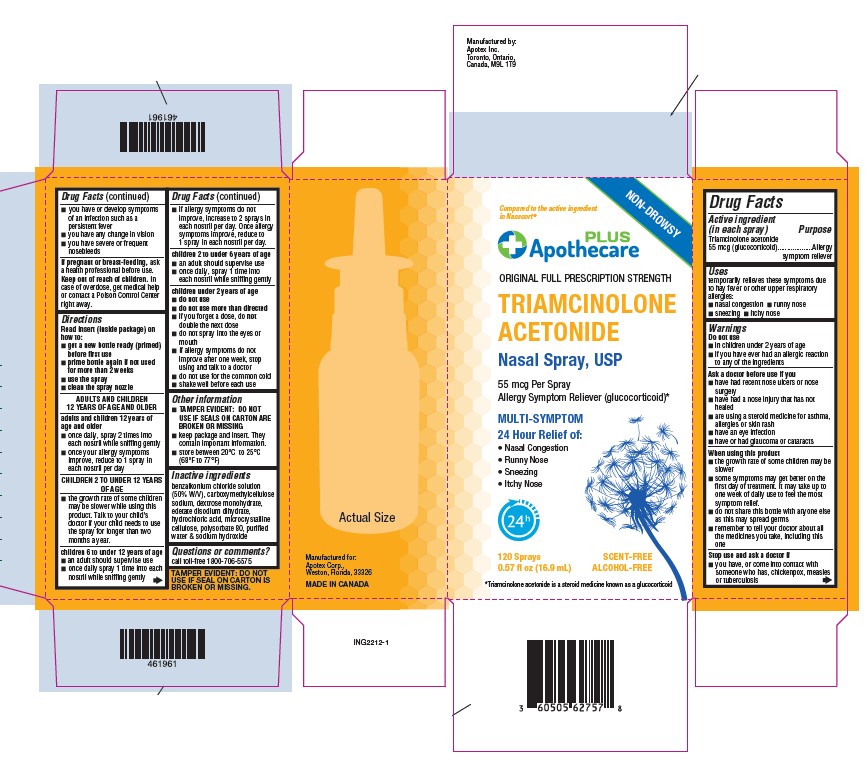
Label: TRIAMCINOLONE ACETONIDE spray, metered
- NDC Code(s): 60505-6275-7
- Packager: Apotex Corp.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active ingredient (in each spray)
Triamcinolone acetonide 55 mcg (glucocorticoid)
-
Purpose
Allergy symptom reliever
-
Uses
Temporarily relieves these symptoms of hey fever or other respiratory allergies: nasal congestion - runny nose - sneezing - itchy nose
-
Warnings
For external use only - Do not use - In children under 2 years of age - If you have ever had an allergic reaction to any of the ingredients - Ask a doctor before use if you - have had ...
-
Directions
Read insert (inside package) on how to: get a new bottle ready (primed) before first use - prime bottle again if not used for more than 2 weeks - use the spray - clean the spray ...
-
Other information
TAMPER EVIDENT: DO NOT USE IF SEALS ON CARTON ARE BROKEN OR MISSING - Store between 20ºC to 25ºC (68ºF to 77ºF)
-
Inactive ingredients
benzalkonium chloride solution (50% W/V) dextrose monohydrate, edetate disodium dihydrate, hydrochloric acid, microcrystalline cellulose, polysorbate 80 purified water & sodium hydroxide ...
-
Questions or comments?
Call toll-free1-800-706-5575
-
Principal Display PanelTriamcinolone Acetonide Nasal Spray, USP - 55 mcg per spray - allergy symptom reliever(glucocorticoid) 120 MD per spray - NDC 60505-6275-7
-
INGREDIENTS AND APPEARANCEProduct Information