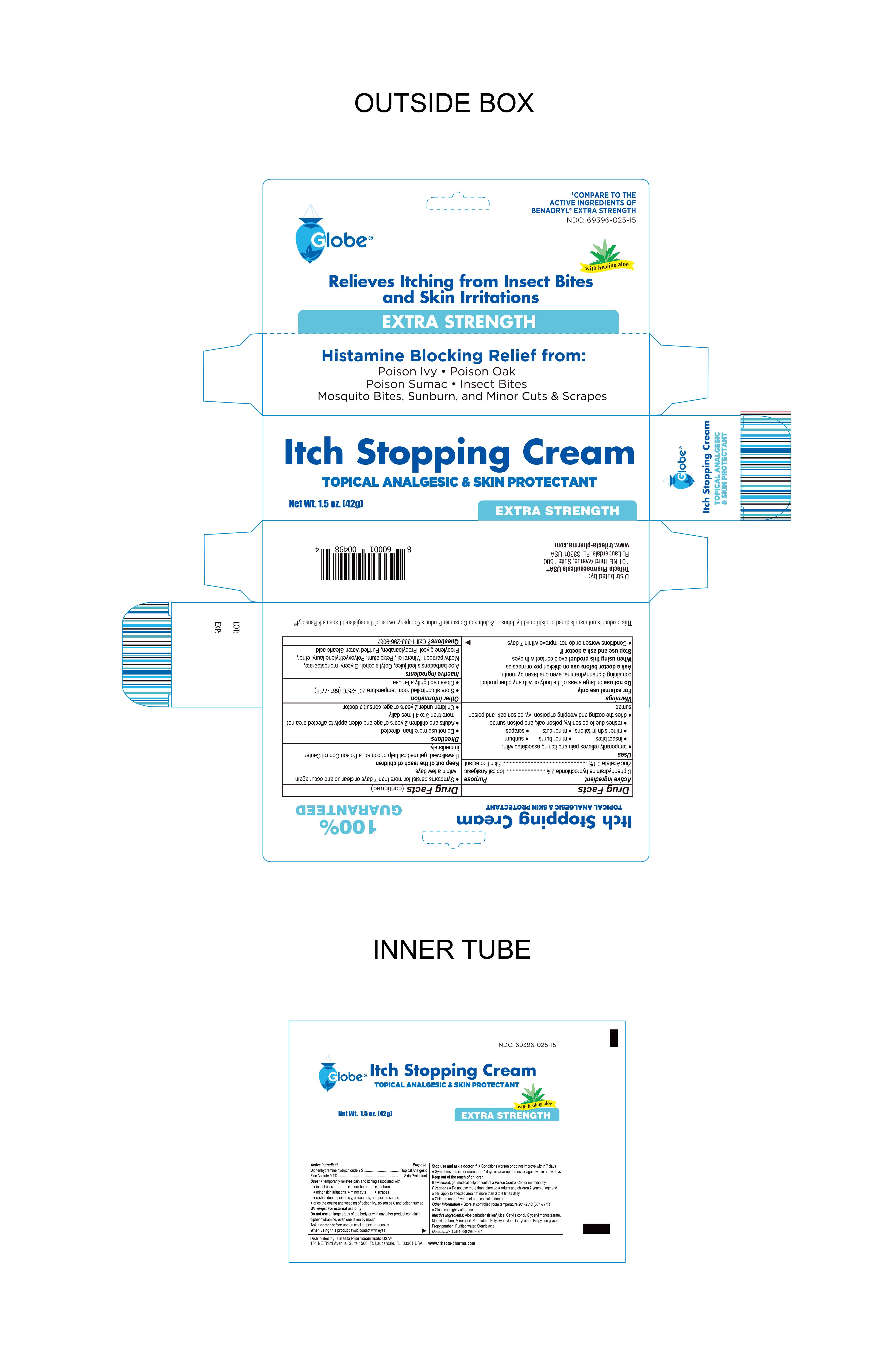
Label: ANTI-ITCH CREAM- diphenhydramine hydrochloride 2%, zinc acetate 0.1% cream
- NDC Code(s): 69396-025-05, 69396-025-15
- Packager: Trifecta Pharmaceuticals USA, LLC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 2, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Active Ingredient
- Purpose
- INDICATIONS & USAGE
-
Warnings
For External Use Only
Do not use on large areas of the body or with any other product containing diphenhydramine, even one taken by mouth
Ask a doctor before use on chicken pox or measles
When using this product avoid contact with eyes
Stop use and ask a doctor if
- Conditions worsen or do not improve within 7 days
- Symptoms persist for more than 7 days or clear up and occur again within a few days
- Stop use and ask a doctor
- Keep out of the reach of children
- Directions
- Other information
- Inactive Ingredients
- Distributed By:
- Packaging
-
INGREDIENTS AND APPEARANCE
ANTI-ITCH CREAM
diphenhydramine hydrochloride 2%, zinc acetate 0.1% creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69396-025 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 2 g in 100 g ZINC ACETATE (UNII: FM5526K07A) (ZINC CATION - UNII:13S1S8SF37) ZINC ACETATE 0.1 g in 100 g Inactive Ingredients Ingredient Name Strength LAURETH-23 (UNII: N72LMW566G) WATER (UNII: 059QF0KO0R) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) PETROLATUM (UNII: 4T6H12BN9U) MINERAL OIL (UNII: T5L8T28FGP) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) STEARIC ACID (UNII: 4ELV7Z65AP) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PROPYLPARABEN (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69396-025-05 1 in 1 BOX 12/29/2016 1 15 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:69396-025-15 1 in 1 BOX 02/11/2020 2 42 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 12/29/2016 Labeler - Trifecta Pharmaceuticals USA, LLC. (079424163)