Label: METOPROLOL SUCCINATE- metoprolol succinate er tablets tablet, film coated, extended release
-
NDC Code(s):
68001-500-00,
68001-500-03,
68001-500-08,
68001-501-00, view more68001-501-03, 68001-501-08, 68001-502-00, 68001-502-03, 68001-502-08, 68001-503-00
- Packager: BluePoint Laboratories
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use METOPROLOL SUCCINATE EXTENDED-RELEASE TABLETS safely and effectively. See full prescribing information for METOPROLOL SUCCINATE ...These highlights do not include all the information needed to use METOPROLOL SUCCINATE EXTENDED-RELEASE TABLETS safely and effectively. See full prescribing information for METOPROLOL SUCCINATE EXTENDED-RELEASE TABLETS.
METOPROLOL SUCCINATE extended-release tablets, for oral use
Initial U.S. Approval: 1992INDICATIONS AND USAGE
Metoprolol succinate, is a beta-adrenergic blocker indicated for the treatment of:
- Hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and non-fatal cardiovascular events, primarily strokes and myocardial infarctions. ( 1.1)
- Angina Pectoris. ( 1.2)
- Heart Failure, to reduce the risk of cardiovascular mortality and heart failure hospitalizations in patients with heart failure. ( 1.3)
DOSAGE AND ADMINISTRATION
- Administer once daily. Titrate at weekly or longer intervals as needed and tolerated. ( 2)
- Hypertension: Starting dose is 25 mg to 100mg. ( 2.1)
- Angina Pectoris: Starting dose is 100 mg. ( 2.2)
- Heart Failure: Starting dose is 12.5 mg or 25mg. ( 2.3)
- Switching from immediate -release metoprolol to metoprolol succinate extended-release tablets: use the same total daily dose of metoprolol succinate extended-release tablets. ( 2)
DOSAGE FORMS AND STRENGTHS
- Metoprolol succinate extended-release tablets: 25 mg, 50 mg, 100 mg, and 200 mg. ( 3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Abrupt cessation may exacerbate myocardial ischemia. ( 5.1)
- Heart Failure: Worsening cardiac failure may occur. ( 5.2)
- Bronchospastic Disease: Avoid beta-blockers. ( 5.3)
- Concomitant use of glycosides, clonidine, diltiazem and verapamil with beta-blockers can increase the risk of bradycardia. ( 5.4)
- Pheochromocytoma: Initiate therapy with an alpha-blocker. ( 5.5)
- Major Surgery: Avoid initiation of high-dose extended-release metoprolol in patients undergoing non-cardiac surgery. Do not routinely withdraw chronic beta-blocker therapy prior to surgery. ( 5.6, 6.1)
- Hypoglycemia: May increase risk for hypoglycemia and mask early warning signs. ( 5.7)
- Thyrotoxicosis: Abrupt withdrawal in patients with thyrotoxicosis might precipitate a thyroid storm. ( 5.8)
- Peripheral Vascular Disease: Can aggravate symptoms of arterial insufficiency. (5.9)
- Patients may be unresponsive to the usual doses of epinephrine used to treat allergic reaction. (5.10)
ADVERSE REACTIONS
- Most common adverse reactions: tiredness, dizziness, depression, shortness of breath, bradycardia, hypotension, diarrhea, pruritus, rash. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Ascend Laboratories, LLC at 1-877-272-7901 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Catecholamine-depleting drugs may have an additive effect when given with beta-blocking agents. ( 7.1)
- CYP2D6 Inhibitors are likely to increase metoprolol concentration. ( 7.2)
- Beta-blockers including metoprolol, may exacerbate the rebound hypertension that can follow the withdrawal of clonidine. ( 7.3)
USE IN SPECIFIC POPULATIONS
- Hepatic Impairment: Consider initiating metoprolol succinate extended-release tablets therapy at low doses and gradually increase dosage to optimize therapy, while monitoring closely for adverse events. (8.6)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 2/2023
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS & USAGE
1.1 Hypertension
1.2 Angina Pectoris
1.3 Heart Failure
2 DOSAGE & ADMINISTRATION
2.1 Hypertension
2.2 Angina Pectoris
2.3 Heart Failure
3 DOSAGE FORMS & STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Abrupt Cessation of Therapy
5.2 Heart Failure
5.3 Bronchospastic Disease
5.5 Pheochromocytoma
5.6 Major Surgery
5.7 Hypoglycemia
5.8 Thyrotoxicosis
5.9 Peripheral Vascular Disease
5.10 Anaphylactic Reaction
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post-Marketing Experience
7 DRUG INTERACTIONS
7.1 Catecholamine Depleting Drugs
7.2 CYP2D6 Inhibitors
7.3 Digitalis, Clonidine, and Calcium Channel Blockers
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.5 Pharmacogenomics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility
14 CLINICAL STUDIES
14.1 Hypertension
14.2 Angina Pectoris
14.3 Heart Failure
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS & USAGE1.1 Hypertension - Metoprolol succinate extended-release tablets are indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure lowers the risk of fatal and ...
1.1 Hypertension
Metoprolol succinate extended-release tablets are indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure lowers the risk of fatal and non-fatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes including metoprolol.
Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than 1 drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program’s Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC).
Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly.
Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal.
Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy.
Metoprolol succinate extended-release tablets may be administered with other antihypertensive agents.
1.2 Angina Pectoris
Metoprolol succinate extended-release tablets are indicated in the long-term treatment of angina pectoris, to reduce angina attacks and to improve exercise tolerance.
Close1.3 Heart Failure
Metoprolol succinate extended-release tablets are indicated to reduce the risk of cardiovascular mortality and heart-failure hospitalization in patients with heart failure.
-
2 DOSAGE & ADMINISTRATION2.1 Hypertension - Adults: The usual initial dosage is 25 mg to 100 mg daily in a single dose. Adjust dosage at weekly (or longer) intervals until optimum blood pressure reduction is achieved ...
2.1 Hypertension
Adults: The usual initial dosage is 25 mg to 100 mg daily in a single dose. Adjust dosage at weekly (or longer) intervals until optimum blood pressure reduction is achieved. In general, the maximum effect of any given dosage level will be apparent after 1 week of therapy. Dosages above 400 mg per day have not been studied.
Pediatric Hypertensive Patients ≥ 6 Years of age: The recommended starting dose of metoprolol succinate extended-release tablets are 1 mg/kg once daily, but the maximum initial dose should not exceed 50 mg once daily. Adjust dosage according to blood pressure response. Doses above 2 mg/kg (or in excess of 200 mg) once daily have not been studied in pediatric patients [see Use in Specific Populations ( 8.4) and Clinical Pharmacology ( 12.3)].
Metoprolol succinate extended-release tablets have not been studied in pediatric patients < 6 years of age [see Use in Specific Populations ( 8.4)] .
2.2 Angina Pectoris
Individualize the dosage of metoprolol succinate extended-release tablets. The usual initial dosage is 100 mg daily, given in a single dose. Gradually increase the dosage at weekly intervals until optimum clinical response has been obtained or there is a pronounced slowing of the heart rate. Dosages above 400 mg per day have not been studied. If treatment is to be discontinued, reduce the dosage gradually over a period of 1 to 2 weeks [ see Warnings and Precautions (5)].
Close2.3 Heart Failure
Dosage must be individualized and closely monitored during up-titration. Prior to initiation of metoprolol succinate extended-release tablets, stabilize the dose of other heart failure drug therapy. The recommended starting dose of metoprolol succinate extended-release tablets are 25 mg once daily for two weeks in patients with NYHA Class II heart failure and 12.5 mg once daily in patients with more severe heart failure. Double the dose every two weeks to the highest dosage level tolerated by the patient or up to 200 mg of metoprolol succinate extended-release tablets. Initial difficulty with titration should not preclude later attempts to introduce metoprolol succinate extended-release tablets. If patients experience symptomatic bradycardia, reduce the dose of metoprolol succinate extended-release tablets. If transient worsening of heart failure occurs, consider treating with increased doses of diuretics, lowering the dose of metoprolol succinate extended-release tablets, or temporarily discontinuing it. The dose of metoprolol succinate extended-release tablets should not be increased until symptoms of worsening heart failure have been stabilized.
-
3 DOSAGE FORMS & STRENGTHSMetoprolol Succinate Extended-Release Tablets, USP are available containing 23.75 mg, 47.5 mg, 95 mg or 190mg of metoprolol succinate, USP equivalent to 25 mg, 50 mg, 100 mg or 200 mg of ...
Metoprolol Succinate Extended-Release Tablets, USP are available containing 23.75 mg, 47.5 mg, 95 mg or 190mg of metoprolol succinate, USP equivalent to 25 mg, 50 mg, 100 mg or 200 mg of metoprolol tartrate, USP, respectively.
25 mg tablets: White to off white, oval shape, biconvex film coated tablet with breakline on one side and debossed with 'A' and '3' on each side of breakline on other side.
50 mg tablets: White to off white, round shape, biconvex film coated tablet with breakline on one side and debossed with 'A50' on other side.
100 mg tablets: White to off white, round shape, biconvex film coated tablet with breakline on one side and debossed with 'A100' on other side.
200 mg tablets: White to off white, oval shape, biconvex film coated tablet with breakline on one side and debossed with 'A200' on other side.
Close -
4 CONTRAINDICATIONSMetoprolol succinate extended-release tablets are contraindicated in severe bradycardia, second- or third-degree heart block, cardiogenic shock, decompensated heart failure, sick sinus syndrome ...
Metoprolol succinate extended-release tablets are contraindicated in severe bradycardia, second- or third-degree heart block, cardiogenic shock, decompensated heart failure, sick sinus syndrome (unless a permanent pacemaker is in place), and in patients who are hypersensitive to any component of this product.
Close -
5 WARNINGS AND PRECAUTIONS5.1 Abrupt Cessation of Therapy - Following abrupt cessation of therapy with certain beta-blocking agents, exacerbations of angina pectoris and, in some cases, myocardial infarction have ...
5.1 Abrupt Cessation of Therapy
Following abrupt cessation of therapy with certain beta-blocking agents, exacerbations of angina pectoris and, in some cases, myocardial infarction have occurred. When discontinuing chronically administered metoprolol succinate extended-release tablets, particularly in patients with ischemic heart disease, gradually reduce the dosage over a period of 1 to 2 weeks and monitor the patient. If angina markedly worsens or acute coronary ischemia develops, promptly reinstate metoprolol succinate extended-release tablets, and take measures appropriate for the management of unstable angina. Warn patients not to interrupt therapy without their physician’s advice. Because coronary artery disease is common and may be unrecognized, avoid abruptly discontinuing metoprolol succinate extended-release tablets in patients treated only for hypertension.
5.2 Heart Failure
Worsening cardiac failure may occur during up-titration of metoprolol succinate extended-release tablets. If such symptoms occur, increase diuretics and restore clinical stability before advancing the dose of metoprolol succinate extended-release tablets [see Dosage and Administration ( 2)]. It may be necessary to lower the dose of metoprolol succinate extended-release tablets or temporarily discontinue it. Such episodes do not preclude subsequent successful titration of metoprolol succinate extended-release tablets.
5.3 Bronchospastic Disease
Patients with bronchospastic diseases should, in general, not receive beta-blockers. Because of its relative beta 1 -cardio-selectivity, however, metoprolol succinate extended-release tablets may be used in patients with bronchospastic disease who do not respond to, or cannot tolerate, other antihypertensive treatment. Because beta 1-selectivity is not absolute, use the lowest possible dose of metoprolol succinate extended-release tablets. Bronchodilators, including beta 2-agonists, should be readily available or administered concomitantly [see Dosage and Administration ( 2)].
5.4 Bradycardia
Bradycardia, including sinus pause, heart block, and cardiac arrest have occurred with the use of metoprolol succinate extended-release tablets. Patients with first-degree atrioventricular block, sinus node dysfunction, conduction disorders (including Wolff-Parkinson-White) or on concomitant drugs that cause bradycardia [see Drug Interactions ( 7.3)], may be at increased risk. Monitor heart rate in patients receiving metoprolol succinate extended-release tablets. If severe bradycardia develops, reduce or stop metoprolol succinate extended-release tablets.
5.5 Pheochromocytoma
If metoprolol succinate extended-release tablets are used in the setting of pheochromocytoma, it should be given in combination with an alpha-blocker, and only after the alpha-blocker has been initiated. Administration of beta-blockers alone in the setting of pheochromocytoma has been associated with a paradoxical increase in blood pressure due to the attenuation of beta-mediated vasodilatation in skeletal muscle.
5.6 Major Surgery
Avoid initiation of a high-dose regimen of extended-release metoprolol in patients undergoing non-cardiac surgery, since such use in patients with cardiovascular risk factors has been associated with bradycardia, hypotension, stroke, and death.
Chronically administered beta-blocking therapy should not be routinely withdrawn prior to major surgery; however, the impaired ability of the heart to respond to reflex adrenergic stimuli may augment the risks of general anesthesia and surgical procedures.
5.7 Hypoglycemia
Beta-blockers may prevent early warning signs of hypoglycemia, such as tachycardia, and increase the risk for severe or prolonged hypoglycemia at any time during treatment, especially in patients with diabetes mellitus or children and patients who are fasting (i.e., surgery, not eating regularly, or are vomiting). If severe hypoglycemia occurs, patients should be instructed to seek emergency treatment.
5.8 Thyrotoxicosis
Beta-adrenergic blockade may mask certain clinical signs of hyperthyroidism, such as tachycardia. Abrupt withdrawal of betablockade may precipitate a thyroid storm.
5.9 Peripheral Vascular Disease
Beta-blockers can precipitate or aggravate symptoms of arterial insufficiency in patients with peripheral vascular disease.
Close5.10 Anaphylactic Reaction
While taking beta-blockers, patients with a history of severe anaphylactic reactions to a variety of allergens may be more reactive to repeated challenge and may be unresponsive to the usual doses of epinephrine used to treat an allergic reaction.
-
6 ADVERSE REACTIONSThe following adverse reactions are described elsewhere in labeling: Worsening angina or myocardial infarction [see Warnings and Precautions ( 5)] ...
The following adverse reactions are described elsewhere in labeling:
- Worsening angina or myocardial infarction [see Warnings and Precautions ( 5)].
- Worsening heart failure [see Warnings and Precautions ( 5)].
- Worsening AV block [see Contraindications ( 4)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The adverse reaction information from clinical trials does, however, provide a basis for identifying the adverse events that appear to be related to drug use and for approximating rates.
Hypertension and Angina: Most adverse reactions have been mild and transient. The most common (>2%) adverse reactions are tiredness, dizziness, depression, diarrhea, shortness of breath, bradycardia, and rash.
Heart Failure: In the MERIT-HF study comparing metoprolol succinate extended-release tablets in daily doses up to 200 mg (mean dose 159 mg once-daily; n=1,990) to placebo (n=2,001), 10.3% of metoprolol succinate extended-release tablets patients discontinued for adverse reactions vs. 12.2% of placebo patients.
The table below lists adverse reactions in the MERIT-HF study that occurred at an incidence of ≥ 1% in the metoprolol succinate extended-release tablets group and greater than placebo by more than 0.5%, regardless of the assessment of causality.
Adverse Reactions Occurring in the MERIT-HF Study at an Incidence ≥1% in the Metoprolol succinate extended-release tablets Group and Greater Than Placebo by More Than 0.5%
Metoprolol Succinate Extended-Release Tablets
n=1,990 % of patients
Placebo
n=2,001 % of patients
Dizziness/vertigo
1.8
1.0
Bradycardia
1.5
0.4Post-operative Adverse Events: In a randomized, double-blind, placebo-controlled trial of 8,351 patients with or at risk for atherosclerotic disease undergoing non-vascular surgery and who were not taking beta–blocker therapy, metoprolol succinate extended-release tablets 100 mg was started 2 to 4 hours prior to surgery then continued for 30 days at 200 mg per day. Metoprolol succinate extended-release tablets use was associated with a higher incidence of bradycardia (6.6% vs. 2.4%; HR 2.74; 95% CI 2.19, 3.43), hypotension (15% vs. 9.7%; HR 1.55; 95% CI 1.37, 1.74), stroke (1.0% vs. 0.5%; HR 2.17; 95% CI 1.26, 3.74) and death (3.1% vs. 2.3%; HR 1.33; 95% CI 1.03, 1.74) compared to placebo.
Close6.2 Post-Marketing Experience
The following adverse reactions have been identified during post-approval use of metoprolol succinate extended-release tablets or immediate-release metoprolol. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiovascular: Cold extremities, arterial insufficiency (usually of the Raynaud type), palpitations, peripheral edema, syncope, chest pain, hypotension.
Respiratory: Wheezing (bronchospasm), dyspnea.
Central Nervous System: Confusion, short-term memory loss, headache, somnolence, nightmares, insomnia, anxiety/nervousness, hallucinations, paresthesia.
Gastrointestinal: Nausea, dry mouth, constipation, flatulence, heartburn, hepatitis, vomiting.
Hypersensitive Reactions: Pruritus.
Miscellaneous: Musculoskeletal pain, arthralgia, blurred vision, decreased libido, male impotence, tinnitus, reversible alopecia, agranulocytosis, dry eyes, worsening of psoriasis, Peyronie’s disease, sweating, photosensitivity, taste disturbance.
Potential Adverse Reactions: In addition, there are adverse reactions not listed above that have been reported with other beta-adrenergic blocking agents and should be considered potential adverse reactions to metoprolol succinate extended-release tablets.
Central Nervous System: Reversible mental depression progressing to catatonia; an acute reversible syndrome characterized by disorientation for time and place, short-term memory loss, emotional lability, clouded sensorium, and decreased performance on neuropsychometrics.
Hematologic: Agranulocytosis, nonthrombocytopenic purpura, thrombocytopenic purpura.
Hypersensitive Reactions: Laryngospasm, respiratory distress. -
7 DRUG INTERACTIONS7.1 Catecholamine Depleting Drugs - Catecholamine depleting drugs (e.g., reserpine, monoamine oxidase (MAO) inhibitors) may have an additive effect when given with beta-blocking agents. Observe ...
7.1 Catecholamine Depleting Drugs
Catecholamine depleting drugs (e.g., reserpine, monoamine oxidase (MAO) inhibitors) may have an additive effect when given with beta-blocking agents. Observe patients treated with metoprolol succinate extended-release tablets plus a catecholamine depletor for evidence of hypotension or marked bradycardia, which may produce vertigo, syncope, or postural hypotension.
7.2 CYP2D6 Inhibitors
Drugs that are strong inhibitors of CYP2D6 such as quinidine, fluoxetine, paroxetine, and propafenone were shown to double metoprolol concentrations. While there is no information about moderate or weak inhibitors, these too are likely to increase metoprolol concentration. Increases in plasma concentration decrease the cardioselectivity of metoprolol [see Clinical Pharmacology ( 12.3)]. Monitor patients closely when the combination cannot be avoided.
Close7.3 Digitalis, Clonidine, and Calcium Channel Blockers
Digitalis glycosides, clonidine, diltiazem, and verapamil slow atrioventricular conduction and decrease heart rate. Concomitant use with beta-blockers can increase the risk of bradycardia.
If clonidine and a beta-blocker, such as metoprolol are co-administered, withdraw the beta-blocker several days before the gradual withdrawal of clonidine because beta-blockers may exacerbate the rebound hypertension that can follow the withdrawal of clonidine. If replacing clonidine by beta-blocker therapy, delay the introduction of beta-blockers for several days after clonidine administration has stopped.
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Untreated hypertension and heart failure during pregnancy can lead to adverse outcomes for the mother and the fetus - (see Clinical Considerations) ...
8.1 Pregnancy
Risk Summary
Untreated hypertension and heart failure during pregnancy can lead to adverse outcomes for the mother and the fetus (see Clinical Considerations). Available data from published observational studies have not demonstrated a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes with metoprolol use during pregnancy. However, there are inconsistent reports of intrauterine growth restriction, preterm birth, and perinatal mortality with maternal use of beta-blockers, including metoprolol, during pregnancy (see Data). In animal reproduction studies, metoprolol has been shown to increase post-implantation loss and decrease neonatal survival in rats at oral dosages of 500 mg/kg/day, approximately 24 times the daily dose of 200 mg in a 60-kg patient on a mg/m2 basis.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical consideration
Disease-associated maternal and/or embryo/fetal risk
Hypertension in pregnancy increases the maternal risk for pre-eclampsia, gestational diabetes, premature delivery, and delivery complications (e.g., need for cesarean section, and post-partum hemorrhage).
Hypertension increases the fetal risk for intrauterine growth restriction and intrauterine death. Pregnant women with hypertension should be carefully monitored and managed accordingly.
Stroke volume and heart rate increase during pregnancy, increasing cardiac output, especially during the first trimester. There is a risk for preterm birth with pregnant women with chronic heart failure in 3rd trimester of pregnancy.
Fetal/Neonatal adverse reactions
Metoprolol crosses the placenta. Neonates born to mothers who are receiving metoprolol during pregnancy, may be at risk for hypotension, hypoglycemia, bradycardia, and respiratory depression. Observe neonates and manage accordingly.
Data
Human Data
Data from published observational studies did not demonstrate an association of major congenital malformations and use of metoprolol in pregnancy. The published literature has reported inconsistent findings of intrauterine growth retardation, preterm birth, and perinatal mortality with maternal use of metoprolol during pregnancy; however, these studies have methodological limitations hindering interpretation. Methodological limitations include retrospective design, concomitant use of other medications, and other unadjusted confounders that may account for the study findings including the underlying disease in the mother. These observational studies cannot definitively establish or exclude any drug-associated risk during pregnancy.
Animal Data
Metoprolol has been shown to increase post-implantation loss and decrease neonatal survival in rats at oral dosages of 500 mg/kg/day, i.e., 24 times, on a mg/m2 basis, the daily dose of 200 mg in a 60-kg patient.
No fetal abnormalities were observed when pregnant rats received metoprolol orally up to a dose of 200 mg/kg/day, i.e., 10 times, the daily dose of 200 mg in a 60-kg patient.
8.2 Lactation
Risk Summary
Limited available data from published literature report that metoprolol is present in human milk. The estimated daily infant dose of metoprolol received from breastmilk ranges from 0.05 mg to less than 1 mg. The estimated relative infant dosage was 0.5% to 2% of the mother's weight-adjusted dosage (see Data). No adverse reactions of metoprolol on the breastfed infant have been identified. There is no information regarding the effects of metoprolol on milk production.
Clinical consideration
Monitoring for adverse reactions
Monitor the breastfed infant for bradycardia and other symptoms of beta-blockade such as listlessness (hypoglycemia).
Data
Based on published case reports, the estimated infant daily dose of metoprolol received from breast milk range from 0.05 mg to less than 1 mg. The estimated relative infant dosage was 0.5% to 2% of the mother's weight adjusted dosage.
In two women who were taking unspecified amount of metoprolol, milk samples were taken after one dose of metoprolol. The estimated amount of metoprolol and alpha-hydroxy metoprolol in breast milk is reported to be less than 2% of the mother's weight adjusted dosage.
In a small study, breast milk was collected every 2 to 3 hours over one dosage interval, in three mothers (at least 3 months postpartum) who took metoprolol of unspecified amount. The average amount of metoprolol present in breast milk was 71.5 mcg/day (range 17.0 to 158.7). The average relative infant dosage was 0.5% of the mother's weight-adjusted dosage.
8.3 Females and Males of Reproductive Potential
Risk Summary
Based on the published literature, beta-blockers (including metoprolol) may cause erectile dysfunction and inhibit sperm motility. No evidence of impaired fertility due to metoprolol was observed in rats [see Nonclinical Toxicology ( 13.1)].
8.4 Pediatric Use
One hundred forty-four hypertensive pediatric patients aged 6 to 16 years were randomized to placebo or to one of three dose levels of metoprolol succinate extended-release tablets (0.2 mg/kg, 1 mg/kg or 2 mg/kg once daily) and followed for 4 weeks. The study did not meet its primary endpoint (dose response for reduction in SBP). Some pre-specified secondary endpoints demonstrated effectiveness including:
• Dose-response for reduction in DBP,
• 1 mg/kg vs. placebo for change in SBP, and
• 2 mg/kg vs. placebo for change in SBP and DBP.
The mean placebo corrected reductions in SBP ranged from 3 to 6 mmHg, and DBP from 1 to 5 mmHg. Mean reduction in heart rate ranged from 5 to 7 bpm but considerably greater reductions were seen in some individuals [ see Dosage and Administration (2.1)] .
No clinically relevant differences in the adverse event profile were observed for pediatric patients aged 6 to 16 years as compared with adult patients.
Safety and effectiveness of metoprolol succinate extended-release tablets have not been established in patients < 6 years of age.
8.5 Geriatric Use
Clinical studies of metoprolol succinate extended-release tablets in hypertension did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience in hypertensive patients has not identified differences in responses between elderly and younger patients.
Of the 1,990 patients with heart failure randomized to metoprolol succinate extended-release tablets in the MERIT-HF trial, 50% (990) were 65 years of age and older and 12% (238) were 75 years of age and older. There were no notable differences in efficacy or the rate of adverse reactions between older and younger patients.
In general, use a low initial starting dose in elderly patients given their greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
8.6 Hepatic Impairment
No studies have been performed with metoprolol succinate extended-release tablets in patients with hepatic impairment. Because metoprolol succinate extended-release tablets are metabolized by the liver, metoprolol blood levels are likely to increase substantially with poor hepatic function. Therefore, initiate therapy at doses lower than those recommended for a given indication; and increase doses gradually in patients with impaired hepatic function.
Close8.7 Renal Impairment
The systemic availability and half-life of metoprolol in patients with renal failure do not differ to a clinically significant degree from those in normal subjects. No reduction in dosage is needed in patients with chronic renal failure [ see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGESigns and Symptoms - Overdosage of metoprolol succinate extended-release tablets may lead to severe bradycardia, hypotension, and cardiogenic shock. Clinical presentation can also include ...
Signs and Symptoms - Overdosage of metoprolol succinate extended-release tablets may lead to severe bradycardia, hypotension, and cardiogenic shock. Clinical presentation can also include atrioventricular block, heart failure, bronchospasm, hypoxia, impairment of consciousness/coma, nausea and vomiting.
Treatment – Consider treating the patient with intensive care. Patients with myocardial infarction or heart failure may be prone to significant hemodynamic instability. Beta-blocker overdose may result in significant resistance to resuscitation with adrenergic agents, including beta-agonists. On the basis of the pharmacologic actions of metoprolol, employ the following measures:
Hemodialysis is unlikely to make a useful contribution to metoprolol elimination [see Clinical Pharmacology ( 12.3)].
Bradycardia: Evaluate the need for atropine, adrenergic-stimulating drugs, or pacemaker to treat bradycardia and conduction disorders.
Hypotension: Treat underlying bradycardia. Consider intravenous vasopressor infusion, such as dopamine or norepinephrine.
Heart failure and shock: May be treated when appropriate with suitable volume expansion, injection of glucagon (if necessary, followed by an intravenous infusion of glucagon), intravenous administration of adrenergic drugs such as dobutamine, with α 1 receptor agonistic drugs added in presence of vasodilation.
Bronchospasm: Can usually be reversed by bronchodilators.
Close -
11 DESCRIPTIONMetoprolol succinate, is a beta 1-selective (cardioselective) adrenoceptor blocking agent, for oral administration, available as extended-release tablets. Metoprolol succinate ...
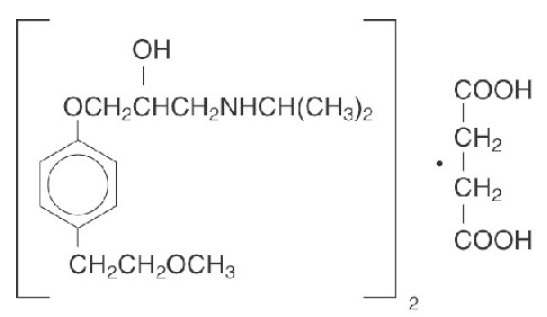 Metoprolol succinate, is a beta
1-selective (cardioselective) adrenoceptor blocking agent, for oral administration, available as extended-release tablets. Metoprolol succinate extended-release tablets, USP has been formulated to provide a controlled and predictable release of metoprolol for once-daily administration. The tablets comprise a multiple unit system containing metoprolol succinate in a multitude of controlled release pellets. Each pellet acts as a separate drug delivery unit and is designed to deliver metoprolol continuously over the dosage interval. The tablets contain 23.75 mg, 47.5 mg, 95 mg and 190 mg of metoprolol succinate equivalent to 25 mg, 50 mg, 100 mg and 200 mg of metoprolol tartrate, USP, respectively. Its chemical name is (±)1- (isopropylamino)-3-[p-(2-methoxyethyl) phenoxy]-2-propanol succinate (2:1) (salt). Its structural formula is
Metoprolol succinate, is a beta
1-selective (cardioselective) adrenoceptor blocking agent, for oral administration, available as extended-release tablets. Metoprolol succinate extended-release tablets, USP has been formulated to provide a controlled and predictable release of metoprolol for once-daily administration. The tablets comprise a multiple unit system containing metoprolol succinate in a multitude of controlled release pellets. Each pellet acts as a separate drug delivery unit and is designed to deliver metoprolol continuously over the dosage interval. The tablets contain 23.75 mg, 47.5 mg, 95 mg and 190 mg of metoprolol succinate equivalent to 25 mg, 50 mg, 100 mg and 200 mg of metoprolol tartrate, USP, respectively. Its chemical name is (±)1- (isopropylamino)-3-[p-(2-methoxyethyl) phenoxy]-2-propanol succinate (2:1) (salt). Its structural formula is
Metoprolol succinate, USP is a white or almost white, crystalline powder with a molecular weight of 652.8. It is freely soluble in water; soluble in methanol; sparingly soluble in ethanol; slightly soluble in Isopropyl alcohol; practically insoluble in ethyl-acetate. Inactive ingredients: Microcrystalline cellulose, Colloidal silicon dioxide, Povidone, Ethyl cellulose, Hypromellose, Methacrylic acid and ethyl acrylate copolymer dispersion, Triethyl citrate, Talc, Silicified Microcrystalline Cellulose, Polyethylene Glycol, Croscarmellose sodium, Sodium Stearyl Fumarate, Titanium dioxide.
“FDA approved dissolution testing specifications differ from USP”
Close -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Metoprolol is a beta - 1-selective (cardioselective) adrenergic receptor blocking agent. This preferential effect is not absolute, however, and at higher ...
12.1 Mechanism of Action
Metoprolol is a beta 1-selective (cardioselective) adrenergic receptor blocking agent. This preferential effect is not absolute, however, and at higher plasma concentrations, metoprolol also inhibits beta 2-adrenoreceptors, chiefly located in the bronchial and vascular musculature.
Metoprolol has no intrinsic sympathomimetic activity, and membrane-stabilizing activity is detectable only at plasma concentrations much greater than required for beta-blockade. Animal and human experiments indicate that metoprolol slows the sinus rate and decreases AV nodal conduction.
The relative beta 1-selectivity of metoprolol has been confirmed by the following: (1) In normal subjects, metoprolol is unable to reverse the beta 2-mediated vasodilating effects of epinephrine. This contrasts with the effect of nonselective beta-blockers, which completely reverse the vasodilating effects of epinephrine. (2) In asthmatic patients, metoprolol reduces FEV 1 and FVC significantly less than a nonselective beta-blocker, propranolol, at equivalent beta 1 -receptor blocking doses.
Hypertension: The mechanism of the antihypertensive effects of beta-blocking agents has not been elucidated. However, several possible mechanisms have been proposed: (1) competitive antagonism of catecholamines at peripheral (especially cardiac) adrenergic neuron sites, leading to decreased cardiac output; (2) a central effect leading to reduced sympathetic outflow to the periphery; and (3) suppression of renin activity.
Angina Pectoris: By blocking catecholamine-induced increases in heart rate, in velocity and extent of myocardial contraction, and in blood pressure, metoprolol reduces the oxygen requirements of the heart at any given level of effort, thus making it useful in the long term management of angina pectoris.
Heart Failure: The precise mechanism for the beneficial effects of beta-blockers in heart failure has not been elucidated.
12.2 Pharmacodynamics
Clinical pharmacology studies have confirmed the beta-blocking activity of metoprolol in man, as shown by (1) reduction in heart rate and cardiac output at rest and upon exercise, (2) reduction of systolic blood pressure upon exercise, (3) inhibition of isoproterenol-induced tachycardia, and (4) reduction of reflex orthostatic tachycardia.
The relationship between plasma metoprolol levels and reduction in exercise heart rate is independent of the pharmaceutical formulation. Beta 1-blocking effects in the range of 30-80% of the maximal effect (approximately 8 to 23% reduction in exercise heart rate) correspond to metoprolol plasma concentrations from 30 to 540 nmol/L. The relative beta 1-selectivity of metoprolol diminishes and blockade of beta 2-adrenoceptors increases at plasma concentration above 300 nmol/L.
In five controlled studies in normal healthy subjects, extended-release metoprolol succinate administered once a day, and immediate-release metoprolol administered once to four times a day, provided comparable total beta 1-blockade over 24 hours (area under the beta 1 -blockade versus time curve) in the dose range 100 to 400 mg. In another controlled study, 50 mg once daily for each product, extended-release metoprolol succinate produced significantly higher total beta 1-blockade over 24 hours than immediate- release metoprolol. For extended-release metoprolol succinate, the percent reduction in exercise heart rate was relatively stable throughout the entire dosage interval and the level of beta 1-blockade increased with increasing doses from 50 to 300 mg daily.
A controlled cross-over study in heart failure patients compared the plasma concentrations and beta 1-blocking effects of 50 mg immediate-release metoprolol administered t.i.d., and 100 mg and 200 mg extended-release metoprolol succinate once daily. Extended-release metoprolol succinate 200 mg once daily produced a larger effect on suppression of exercise-induced and Holter-monitored heart rate over 24 hours compared to 50 mg t.i.d. of immediate-release metoprolol.
In other studies, treatment with metoprolol succinate produced an improvement in left ventricular ejection fraction. Metoprolol succinate was also shown to delay the increase in left ventricular end-systolic and end-diastolic volumes after 6 months of treatment.
Although beta-adrenergic receptor blockade is useful in the treatment of angina, hypertension, and heart failure there are situations in which sympathetic stimulation is vital. In patients with severely damaged hearts, adequate ventricular function may depend on sympathetic drive. In the presence of AV block, beta-blockade may prevent the necessary facilitating effect of sympathetic activity on conduction. Beta 2-adrenergic blockade results in passive bronchial constriction by interfering with endogenous adrenergic bronchodilator activity in patients subject to bronchospasm and may also interfere with exogenous bronchodilators in such patients.
12.3 Pharmacokinetics
Absorption
The peak plasma levels following once-daily administration of metoprolol succinate extended-release tablets average one-fourth to one-half the peak plasma levels obtained following a corresponding dose of conventional metoprolol, administered once daily or in divided doses. At steady state the average bioavailability of metoprolol following administration of metoprolol succinate extended-release tablets, across the dosage range of 50 to 400 mg once daily, was 77% relative to the corresponding single or divided doses of conventional metoprolol.
The bioavailability of metoprolol shows a dose-related, although not directly proportional, increase with dose and is not significantly affected by food following metoprolol succinate extended-release tablets administration.
The peak plasma levels following oral administration of conventional metoprolol tablets, however, approximate 50% of levels following intravenous administration, indicating about 50% first-pass metabolism.
Distribution
Metoprolol crosses the blood-brain barrier and has been reported in the CSF in a concentration 78% of the simultaneous plasma concentration. Only a small fraction of the drug (about 12%) is bound to human serum albumin.
Metabolism
Metoprolol is a racemic mixture of R- and S- enantiomers and is primarily metabolized by CYP2D6. When administered orally, it exhibits stereoselective metabolism that is dependent on oxidation phenotype.
Elimination
Elimination is mainly by biotransformation in the liver, and the plasma half-life ranges from approximately 3 to 7 hours. Less than 5% of an oral dose of metoprolol is recovered unchanged in the urine; the rest is excreted by the kidneys as metabolites that appear to have no beta-blocking activity.
Following intravenous administration of metoprolol, the urinary recovery of unchanged drug is approximately 10%.
Specific Populations
Patients with Renal Impairment
The systemic availability and half-life of metoprolol in patients with renal failure do not differ to a clinically significant degree from those in normal subjects.
Pediatric Patients
The pharmacokinetic profile of metoprolol succinate extended-release tablets were studied in 120 pediatric hypertensive patients (6-17 years of age) receiving doses ranging from 12.5 mg to 200 mg once daily. The pharmacokinetics of metoprolol were similar to those described previously in adults. Metoprolol pharmacokinetics have not been investigated in patients < 6 years of age.
Body Weight, Age, and Race
Metoprolol apparent oral clearance (CL/F) increased linearly with body weight. Age, gender, and race had no significant effects on metoprolol pharmacokinetics.
Drug Interactions
CYP2D6
Metoprolol is metabolized predominantly by CYP2D6. In healthy subjects with CYP2D6 extensive metabolizer phenotype, coadministration of quinidine 100 mg, a potent CYP2D6 inhibitor, and immediate-release metoprolol 200 mg tripled the concentration of S-metoprolol and doubled the metoprolol elimination half-life. In four patients with cardiovascular disease, coadministration of propafenone 150 mg t.i.d. with immediate-release metoprolol 50 mg t.i.d. resulted in steady-state concentration of metoprolol 2- to 5-fold what is seen with metoprolol alone. Extensive metabolizers who concomitantly use CYP2D6 inhibiting drugs will have increased (several-fold) metoprolol blood levels, decreasing metoprolol's cardioselectivity [see Drug Interactions ( 7.2)] .
Close12.5 Pharmacogenomics
CYP2D6 is absent in about 8% of Caucasians (poor metabolizers) and about 2% of most other populations. CYP2D6 can be inhibited by several drugs. Poor metabolizers of CYP2D6 will have increased (several-fold) metoprolol blood levels, decreasing metoprolol's cardioselectivity.
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility - Long-term studies in animals have been conducted to evaluate the carcinogenic potential of metoprolol tartrate. In 2-year studies in ...Close
13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility
Long-term studies in animals have been conducted to evaluate the carcinogenic potential of metoprolol tartrate. In 2-year studies in rats at three oral dosage levels of up to 800 mg/kg/day (41 times, on a mg/m 2 basis, the daily dose of 200 mg for a 60-kg patient), there was no increase in the development of spontaneously occurring benign or malignant neoplasms of any type. The only histologic changes that appeared to be drug related were an increased incidence of generally mild focal accumulation of foamy macrophages in pulmonary alveoli and a slight increase in biliary hyperplasia. In a 21-month study in Swiss albino mice at three oral dosage levels of up to 750 mg/kg/day (18 times, on a mg/m2 basis, the daily dose of 200 mg for a 60-kg patient), benign lung tumors (small adenomas) occurred more frequently in female mice receiving the highest dose than in untreated control animals. There was no increase in malignant or total (benign plus malignant) lung tumors, nor in the overall incidence of tumors or malignant tumors. This 21-month study was repeated in CD-1 mice, and no statistically or biologically significant differences were observed between treated and control mice of either sex for any type of tumor.
All genotoxicity tests performed on metoprolol tartrate (a dominant lethal study in mice, chromosome studies in somatic cells, a Salmonella/mammalian-microsome mutagenicity test, and a nucleus anomaly test in somatic interphase nuclei) and metoprolol succinate (a Salmonella/mammalian-microsome mutagenicity test) were negative.
No evidence of impaired fertility due to metoprolol tartrate was observed in a study performed in rats at doses up to 22 times, on a mg/m 2 basis, the daily dose of 200 mg in a 60-kg patient.
-
14 CLINICAL STUDIES 14.1 Hypertension - In a double-blind study, 1092 patients with mild-to-moderate hypertension were randomized to once daily metoprolol succinate extended-release tablets (25 mg, 100 mg, or 400 ...
14.1 Hypertension
In a double-blind study, 1092 patients with mild-to-moderate hypertension were randomized to once daily metoprolol succinate extended-release tablets (25 mg, 100 mg, or 400 mg), PLENDIL ® (felodipine extended-release tablets), the combination, or placebo. After 9 weeks, metoprolol succinate extended-release tablets alone decreased sitting blood pressure by 6-8/4-7 mmHg (placebo-corrected change from baseline) at 24 hours post-dose. The combination of metoprolol succinate extended-release tablets with PLENDIL has greater effects on blood pressure.
In controlled clinical studies, an immediate-release dosage form of metoprolol was an effective antihypertensive agent when used alone or as concomitant therapy with thiazide-type diuretics at dosages of 100 mg to 450 mg daily. Metoprolol succinate extended-release tablets, in dosages of 100 mg to 400 mg once daily, produces similar β 1-blockade as conventional metoprolol tablets administered two to four times daily. In addition, metoprolol succinate extended-release tablets administered at a dose of 50 mg once daily lowered blood pressure 24-hours post-dosing in placebo-controlled studies. In controlled, comparative, clinical studies, immediate-release metoprolol appeared comparable as an antihypertensive agent to propranolol, methyldopa, and thiazide-type diuretics, and affected both supine and standing blood pressure. Because of variable plasma levels attained with a given dose and lack of a consistent relationship of antihypertensive activity to drug plasma concentration, selection of proper dosage requires individual titration.
14.2 Angina Pectoris
In controlled clinical trials, an immediate-release formulation of metoprolol has been shown to be an effective antianginal agent, reducing the number of angina attacks and increasing exercise tolerance. The dosage used in these studies ranged from 100 to 400 mg daily. Metoprolol succinate extended-release tablets, in dosages of 100 to 400 mg once daily, has been shown to possess beta-blockade similar to conventional metoprolol tablets administered two to four times daily.
Close14.3 Heart Failure
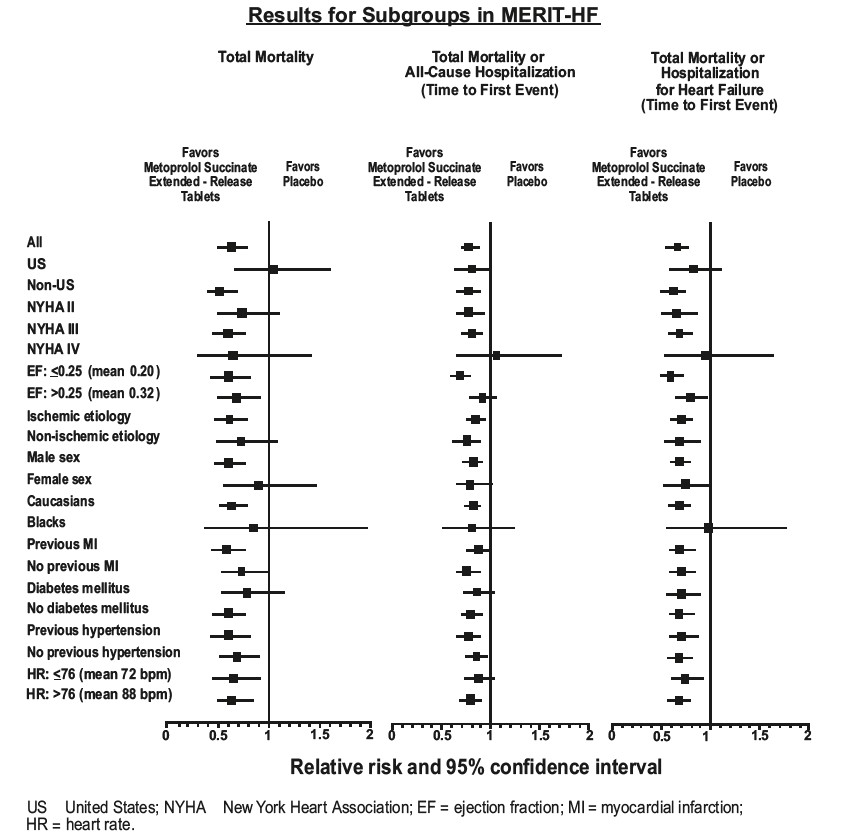 MERIT-HF was a randomized double-blind, placebo-controlled study of metoprolol succinate extended-release tablets in which 3991 patients with ejection fraction ≤0.40 and NYHA Class II-IV heart failure attributable to ischemia, hypertension, or cardiomyopathy were randomized 1:1 to metoprolol succinate extended-release tablets or placebo. The protocol excluded patients with contraindications to betablocker use, those expected to undergo heart surgery, and those within 28 days of myocardial infarction or unstable angina. The primary endpoints of the trial were (1) all-cause mortality plus all-cause hospitalization (time to first event) and (2) all-cause mortality. Patients were stabilized on optimal concomitant therapy for heart failure, including diuretics, ACE inhibitors, cardiac glycosides, and nitrates. At randomization, 41% of patients were NYHA Class II; 55% NYHA Class III; 65% of patients had heart failure attributed to ischemic heart disease; 44% had a history of hypertension; 25% had diabetes mellitus; 48% had a history of myocardial infarction. Among patients in the trial, 90% were on diuretics, 89% were on ACE inhibitors, 64% were on digitalis, 27% were on a lipid-lowering agent, 37% were on an oral anticoagulant, and the mean ejection fraction was 0.28. The mean duration of follow-up was one year. At the end of the study, the mean daily dose of metoprolol succinate extended-release tablets were 159 mg.
MERIT-HF was a randomized double-blind, placebo-controlled study of metoprolol succinate extended-release tablets in which 3991 patients with ejection fraction ≤0.40 and NYHA Class II-IV heart failure attributable to ischemia, hypertension, or cardiomyopathy were randomized 1:1 to metoprolol succinate extended-release tablets or placebo. The protocol excluded patients with contraindications to betablocker use, those expected to undergo heart surgery, and those within 28 days of myocardial infarction or unstable angina. The primary endpoints of the trial were (1) all-cause mortality plus all-cause hospitalization (time to first event) and (2) all-cause mortality. Patients were stabilized on optimal concomitant therapy for heart failure, including diuretics, ACE inhibitors, cardiac glycosides, and nitrates. At randomization, 41% of patients were NYHA Class II; 55% NYHA Class III; 65% of patients had heart failure attributed to ischemic heart disease; 44% had a history of hypertension; 25% had diabetes mellitus; 48% had a history of myocardial infarction. Among patients in the trial, 90% were on diuretics, 89% were on ACE inhibitors, 64% were on digitalis, 27% were on a lipid-lowering agent, 37% were on an oral anticoagulant, and the mean ejection fraction was 0.28. The mean duration of follow-up was one year. At the end of the study, the mean daily dose of metoprolol succinate extended-release tablets were 159 mg.
The trial was terminated early for a statistically significant reduction in all-cause mortality (34%, nominal p= 0.00009). The risk of all-cause mortality plus all-cause hospitalization was reduced by 19% (p= 0.00012). The trial also showed improvements in heart failure-related mortality and heart failure-related hospitalizations, and NYHA functional class.
The table below shows the principal results for the overall study population. The figure below illustrates principal results for a wide variety of subgroup comparisons, including US vs. non-US populations (the latter of which was not pre- specified).The combined endpoints of all-cause mortality plus all-cause hospitalization and of mortality plus heart failure hospitalization showed consistent effects in the overall study population and the subgroups. Nonetheless, subgroup analyses can be difficult to interpret, and it is not known whether these represent true differences or chance effects.
Clinical Endpoints in the MERIT-HF StudyClinical Endpoint
Number of Patients
Relative Risk (95% Cl)
Risk Reduction With Metoprolol Succinate Extended-Release Tablets
Nominal P- value
Placebo n=2,001
Metoprolol Succinate Extended-Release Tablets n=1,990
All-cause mortality plus all-caused hospitalization*
767
641
0.81(0.73- 0.90)
19%
0.00012
All-cause mortality
217
145
0.66(0.53- 0.81)
34%
0.00009
All-cause mortality plus heart failure hospitalization*
439
311
0.69(0.60- 0.80)
31%
0.0000008
Cardiovascular mortality
203
128
0.62(0.50- 0.78)
38%
0.000022
Sudden death
132
79
0.59(0.45- 0.78)
41%
0.0002
Death due to worsening heart failure
58
30
0.51(0.33- 0.79)
49%
0.0023
Hospitalizations due to worsening heart failure†
451
317
N/A
N/A
0.0000076
Cardiovascular hospitalization†
773
649
N/A
N/A
0.00028
* Time to first event
† Comparison of treatment groups examines the number of hospitalizations (Wilcoxon test); relative risk and risk reduction are not applicable.
-
16 HOW SUPPLIED/STORAGE AND HANDLINGMetoprolol Succinate Extended-Release Tablets, USP are available containing 23.75 mg, 47.5 mg, 95 mg or 190 mg of metoprolol succinate, USP equivalent to 25 mg, 50 mg, 100 mg or 200 mg of ...
Metoprolol Succinate Extended-Release Tablets, USP are available containing 23.75 mg, 47.5 mg, 95 mg or 190 mg of metoprolol succinate, USP equivalent to 25 mg, 50 mg, 100 mg or 200 mg of metoprolol tartrate, USP, respectively.
25 mg tablets: White to off white, oval shape, biconvex film coated tablet with breakline on one side and debossed with 'A' and '3' on each side of breakline on other side. They are available as follows:
Bottles of 100 tablets 68001-500-00
Bottles of 500 tablets 68001-500-03
Bottle of 1000 tablets 68001-500-08
50 mg tablets: White to off white, round shape, biconvex film coated tablet with breakline on one side and debossed with 'A50' on other side. They are available as follows:
Bottles of 100 tablets 68001-501-00
Bottles of 500 tablets 68001-501-03
Bottle of 1000 tablets 68001-501-08
100 mg tablets: White to off white, round shape, biconvex film coated tablet with breakline on one side and debossed with 'A100' on other side. They are available as follows:
Bottles of 100 tablets 68001-502-00
Bottles of 500 tablets 68001-502-03
Bottle of 1000 tablets 68001-502-08
200 mg tablets: White to off white, oval shape, biconvex film coated tablet with breakline on one side and debossed with 'A200' on other side. They are available as follows:
Bottles of 100 tablets 68001-503-00
Close
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F). [See USP Controlled Room Temperature.] -
17 PATIENT COUNSELING INFORMATIONAdvise patients to take metoprolol succinate extended-release tablets regularly and continuously, as directed, preferably with or immediately following meals. If a dose is missed, the patient ...
Advise patients to take metoprolol succinate extended-release tablets regularly and continuously, as directed, preferably with or immediately following meals. If a dose is missed, the patient should take only the next scheduled dose (without doubling it). Patients should not interrupt or discontinue metoprolol succinate extended-release tablets without consulting the physician.
Advise patients (1) to avoid operating automobiles and machinery or engaging in other tasks requiring alertness until the patient’s response to therapy with metoprolol succinate extended-release tablets has been determined; (2) to contact the physician if any difficulty in breathing occurs; (3) to inform the physician or dentist before any type of surgery that he or she is taking metoprolol succinate extended-release tablets.
Heart failure patients should be advised to consult their physician if they experience signs or symptoms of worsening heart failure such as weight gain or increasing shortness of breath.
Risk of hypoglycemia
Inform patients or caregivers that there is a risk of hypoglycemia when metoprolol succinate extended-release tablets are given to patients who are fasting or who are vomiting. Instruct patients or caregivers how to monitor for signs of hypoglycemia [see Warnings and Precautions ( 5.7)].
The brands listed are trademarks of their respective owners.
Close
Manufactured by:
Alkem Laboratories Ltd.
INDIA
For BluePoint Laboratories
Revised: March/2023 -
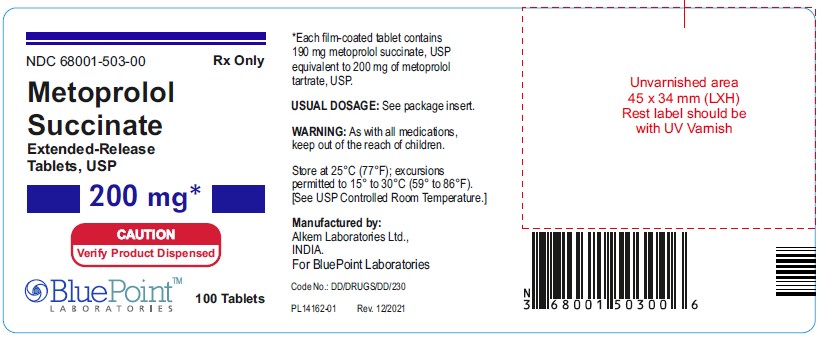
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 68001-500-00 25mg 100 Tablets Rx Only
-
Package/Label Display Panel
NDC 68001-501-00 50mg 100 Tablets Rx Only
-
Package/Label Display Panel
NDC 68001-502-00 100mg 100 Tablets Rx Only
-
Package/Label Display Panel
NDC 68001-503-00 200mg 100 Tablets Rx Only
-
INGREDIENTS AND APPEARANCEProduct Information
METOPROLOL SUCCINATE metoprolol succinate er tablets tablet, film coated, extended release Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:68001-500 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METOPROLOL SUCCINATE (UNII: TH25PD4CCB) (METOPROLOL - UNII:GEB06NHM23) METOPROLOL TARTRATE 25 mg Inactive Ingredients Ingredient Name Strength POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ETHYLCELLULOSE (20 MPA.S) (UNII: BJG0S321QY) METHYLENE CHLORIDE (UNII: 588X2YUY0A) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) TALC (UNII: 7SEV7J4R1U) POLYETHYLENE GLYCOL 6000 (UNII: 30IQX730WE) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) ISOPROPYL ALCOHOL (UNII: ND2M416302) WATER (UNII: 059QF0KO0R) METHACRYLIC ACID AND ETHYL ACRYLATE COPOLYMER (UNII: NX76LV5T8J) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white (off white) Score 2 pieces Shape OVAL Size 12mm Flavor Imprint Code A;3 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68001-500-00 100 in 1 BOTTLE; Type 0: Not a Combination Product 05/14/2021 05/15/2021 2 NDC:68001-500-03 500 in 1 BOTTLE; Type 0: Not a Combination Product 05/14/2021 05/15/2021 3 NDC:68001-500-08 1000 in 1 BOTTLE; Type 0: Not a Combination Product 05/14/2021 05/15/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211143 05/14/2021 05/15/2021 METOPROLOL SUCCINATE metoprolol succinate er tablets tablet, film coated, extended release Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:68001-501 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METOPROLOL SUCCINATE (UNII: TH25PD4CCB) (METOPROLOL - UNII:GEB06NHM23) METOPROLOL TARTRATE 50 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) ETHYLCELLULOSE (20 MPA.S) (UNII: BJG0S321QY) METHYLENE CHLORIDE (UNII: 588X2YUY0A) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) TALC (UNII: 7SEV7J4R1U) POLYETHYLENE GLYCOL 6000 (UNII: 30IQX730WE) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) ISOPROPYL ALCOHOL (UNII: ND2M416302) WATER (UNII: 059QF0KO0R) METHACRYLIC ACID AND ETHYL ACRYLATE COPOLYMER (UNII: NX76LV5T8J) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white (off white) Score 2 pieces Shape ROUND Size 11mm Flavor Imprint Code A50 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68001-501-00 100 in 1 BOTTLE; Type 0: Not a Combination Product 05/14/2021 2 NDC:68001-501-03 500 in 1 BOTTLE; Type 0: Not a Combination Product 05/14/2021 3 NDC:68001-501-08 1000 in 1 BOTTLE; Type 0: Not a Combination Product 05/14/2021 05/15/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211143 05/14/2021 METOPROLOL SUCCINATE metoprolol succinate er tablets tablet, film coated, extended release Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:68001-502 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METOPROLOL SUCCINATE (UNII: TH25PD4CCB) (METOPROLOL - UNII:GEB06NHM23) METOPROLOL TARTRATE 100 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) ETHYLCELLULOSE (20 MPA.S) (UNII: BJG0S321QY) METHYLENE CHLORIDE (UNII: 588X2YUY0A) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) TALC (UNII: 7SEV7J4R1U) POLYETHYLENE GLYCOL 6000 (UNII: 30IQX730WE) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) ISOPROPYL ALCOHOL (UNII: ND2M416302) WATER (UNII: 059QF0KO0R) METHACRYLIC ACID AND ETHYL ACRYLATE COPOLYMER (UNII: NX76LV5T8J) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white (off white) Score 2 pieces Shape ROUND Size 11mm Flavor Imprint Code A100 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68001-502-00 100 in 1 BOTTLE; Type 0: Not a Combination Product 05/14/2021 2 NDC:68001-502-03 500 in 1 BOTTLE; Type 0: Not a Combination Product 05/14/2021 3 NDC:68001-502-08 1000 in 1 BOTTLE; Type 0: Not a Combination Product 05/14/2021 05/15/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211143 05/14/2021 METOPROLOL SUCCINATE metoprolol succinate er tablets tablet, film coated, extended release Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:68001-503 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METOPROLOL SUCCINATE (UNII: TH25PD4CCB) (METOPROLOL - UNII:GEB06NHM23) METOPROLOL TARTRATE 200 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) ETHYLCELLULOSE (20 MPA.S) (UNII: BJG0S321QY) METHYLENE CHLORIDE (UNII: 588X2YUY0A) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) TALC (UNII: 7SEV7J4R1U) POLYETHYLENE GLYCOL 6000 (UNII: 30IQX730WE) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) ISOPROPYL ALCOHOL (UNII: ND2M416302) WATER (UNII: 059QF0KO0R) METHACRYLIC ACID AND ETHYL ACRYLATE COPOLYMER (UNII: NX76LV5T8J) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white (off white) Score 2 pieces Shape OVAL Size 17mm Flavor Imprint Code A200 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68001-503-00 100 in 1 BOTTLE; Type 0: Not a Combination Product 05/14/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211143 05/14/2021 Labeler - BluePoint Laboratories (985523874)
CloseEstablishment Name Address ID/FEI Business Operations Alkem Laboratories Limited 915628612 manufacture(68001-500, 68001-501, 68001-502, 68001-503)
Find additional resources
(also available in the left menu)Safety
Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
METOPROLOL SUCCINATE- metoprolol succinate er tablets tablet, film coated, extended release
Number of versions: 7
RxNorm
METOPROLOL SUCCINATE- metoprolol succinate er tablets tablet, film coated, extended release
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 866412 | metoprolol succinate 100 MG 24HR Extended Release Oral Tablet | PSN |
| 2 | 866412 | 24 HR metoprolol succinate 100 MG Extended Release Oral Tablet | SCD |
| 3 | 866412 | 24 HR metoprolol succinate 100 MG (as metoprolol succinate 95 MG equivalent to 100 MG metoprolol tartrate) Extended Release Oral Tablet | SY |
| 4 | 866412 | metoprolol succinate 100 MG 24 HR Extended Release Oral Tablet | SY |
| 5 | 866419 | metoprolol succinate 200 MG 24HR Extended Release Oral Tablet | PSN |
| 6 | 866419 | 24 HR metoprolol succinate 200 MG Extended Release Oral Tablet | SCD |
| 7 | 866419 | 24 HR metoprolol succinate 200 MG (as metoprolol succinate 190 MG equivalent to 200 MG metoprolol tartrate) Extended Release Oral Tablet | SY |
| 8 | 866419 | metoprolol succinate 200 MG 24 HR Extended Release Oral Tablet | SY |
| 9 | 866427 | metoprolol succinate 25 MG 24HR Extended Release Oral Tablet | PSN |
| 10 | 866427 | 24 HR metoprolol succinate 25 MG Extended Release Oral Tablet | SCD |
| 11 | 866427 | 24 HR metoprolol succinate 25 MG (as metoprolol succinate 23.75 MG equivalent to 25 MG metoprolol tartrate) Extended Release Oral Tablet | SY |
| 12 | 866427 | metoprolol succinate 25 MG 24 HR Extended Release Oral Tablet | SY |
| 13 | 866436 | metoprolol succinate 50 MG 24HR Extended Release Oral Tablet | PSN |
| 14 | 866436 | 24 HR metoprolol succinate 50 MG Extended Release Oral Tablet | SCD |
| 15 | 866436 | 24 HR metoprolol succinate 50 MG (as metoprolol succinate 47.5 MG equivalent to 50 MG metoprolol tartrate) Extended Release Oral Tablet | SY |
| 16 | 866436 | metoprolol succinate 50 MG 24 HR Extended Release Oral Tablet | SY |
Get Label RSS Feed for this Drug
METOPROLOL SUCCINATE- metoprolol succinate er tablets tablet, film coated, extended release
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=6588d77c-090f-4722-9c91-437887d4624a
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
METOPROLOL SUCCINATE- metoprolol succinate er tablets tablet, film coated, extended release
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 68001-500-00 |
| 2 | 68001-500-03 |
| 3 | 68001-500-08 |
| 4 | 68001-501-00 |
| 5 | 68001-501-03 |
| 6 | 68001-501-08 |
| 7 | 68001-502-00 |
| 8 | 68001-502-03 |
| 9 | 68001-502-08 |
| 10 | 68001-503-00 |





 NDC 68001-500-00
NDC 68001-500-00
 NDC 68001-501-00
NDC 68001-501-00
 NDC 68001-502-00
NDC 68001-502-00
 NDC 68001-503-00
NDC 68001-503-00