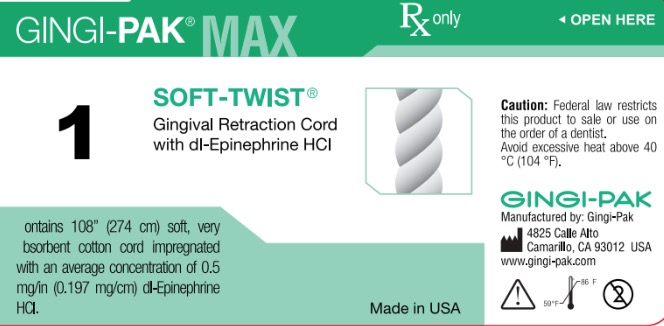
Label: GINGI-PAK MAX SOFT-TWIST NO 1- dl epinephrine hci solution
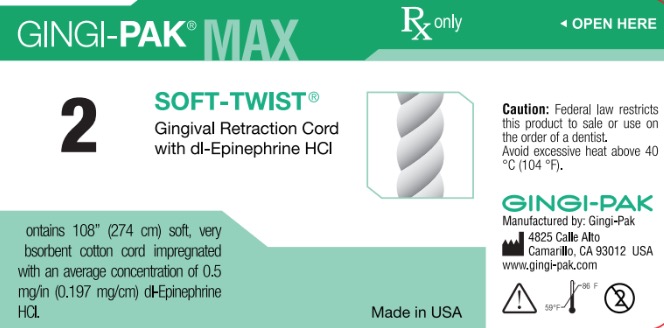
GINGI-PAK MAX SOFT-TWIST NO 2- dl epinephrine hci solution
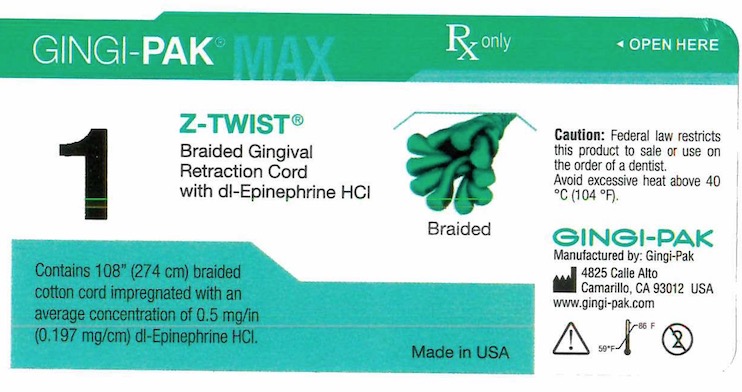
GINGI-PAK MAX Z-TWIST NO 1- dl epinephrine hci solution
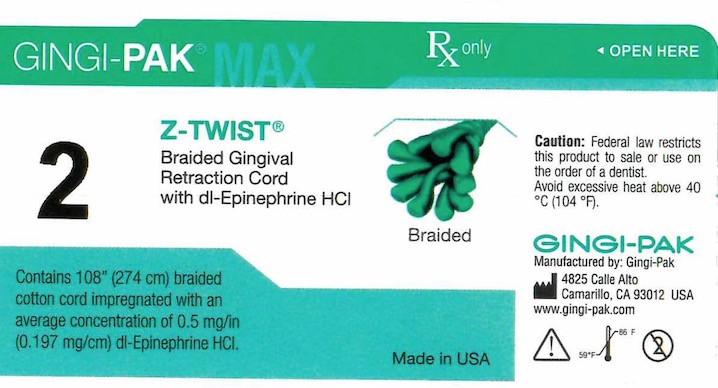
GINGI-PAK MAX Z-TWIST NO 2- dl epinephrine hci solution
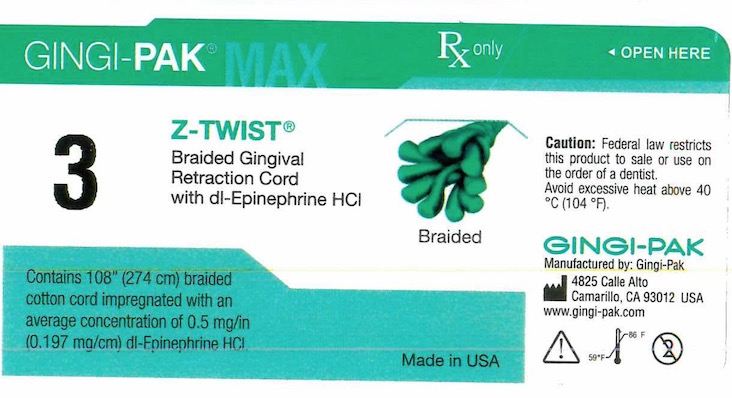
GINGI-PAK MAX Z-TWIST NO 3- dl epinephrine hci solution
GINGI-PAK MAX 2-PLY- dl epinephrine hci solution
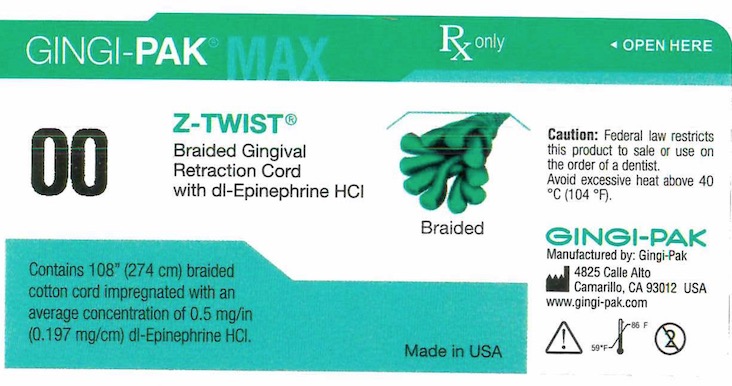
GINGI-PAK MAX Z-TWIST NO 00- dl epinephrine hci solution
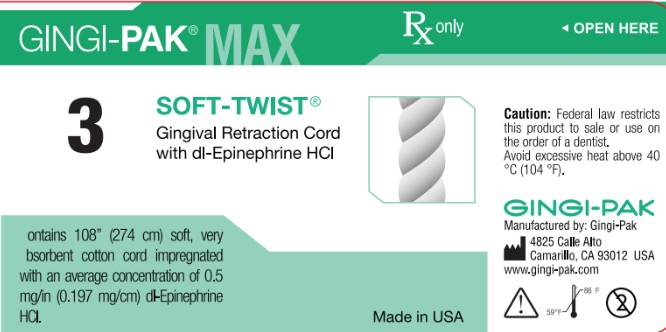
GINGI-PAK MAX SOFT-TWIST NO 3- dl epinephrine hci solution
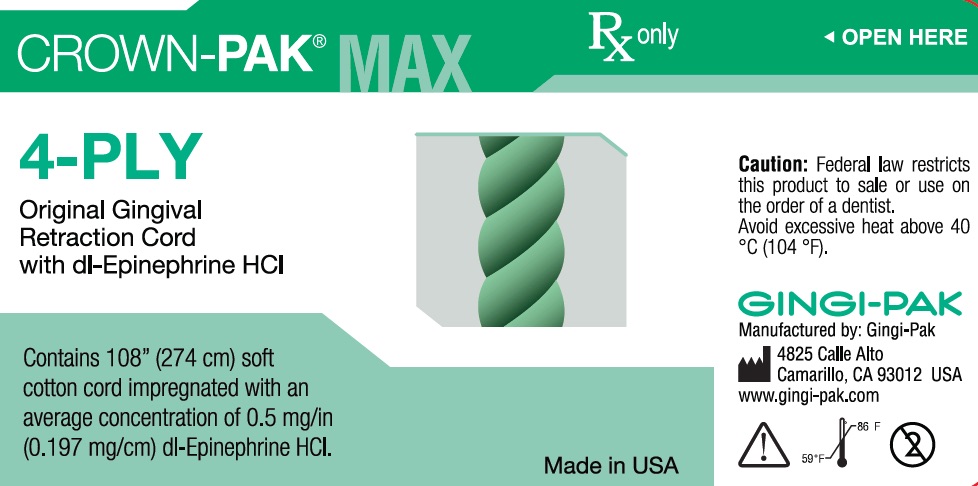
CROWN-PAK MAX 4-PLY- dl epinephrine hci solution
GINGI-PAK COTTON COIL- dl epinephrine hci solution
GINGI-PAK PELLETS- dl epinephrine hci solution
-
NDC Code(s):
10129-001-03,
10129-002-03,
10129-003-03,
10129-004-03, view more10129-005-03, 10129-007-02, 10129-008-01, 10129-051-01, 10129-052-01, 10129-053-01, 10129-054-01
- Packager: Gingi-Pak a Division of the Belport
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 21, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Ask a doctor
- Do not use
- Keep out of reach of children
- Pregnancy warning
- Purpose
- Storage
- Precautions
- Inactive ingredient
- Uses- Gingi-Pak Z-Twist
- Direction
- For topical use only
- Uses- Gingi-Pak Soft-Twist
- Uses- Gingi-Pak 2-ply
- Uses- Crown-Pak 4-ply
- Principal display- Gingi-Pak No 00
- Principal display- Gingi-Pak No 1
- Principal display- Gingi-Pak No 2
- Principal display- Gingi-Pak No 3
- Principal display-Soft-Twist NO1
- Principal display-Soft-Twist NO2
- Principal display-Soft-Twist NO3
- Principal display-2-ply
- Principal display-4-ply
- Principal display-Gingi-Pak Cotton Coil
- Principal display-Gingi-Pak Pellets
-
INGREDIENTS AND APPEARANCE
GINGI-PAK MAX SOFT-TWIST NO 1
dl epinephrine hci solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10129-001 Route of Administration DENTAL, SUBGINGIVAL, PERIODONTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPINEPHRINE HYDROCHLORIDE (UNII: WBB047OO38) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 0.197 mg in 10 mm Inactive Ingredients Ingredient Name Strength COTTON FIBER (UNII: 70LDW53ROO) 10 mm in 10 mm SODIUM DITHIONITE (UNII: 2K5B8F6ES1) 6.15 ug in 10 mm EDETIC ACID (UNII: 9G34HU7RV0) 0.033 ug in 10 mm METHYLPARABEN (UNII: A2I8C7HI9T) 1.65 ug in 10 mm PROPYLPARABEN (UNII: Z8IX2SC1OH) 0.813 ug in 10 mm SODIUM CHLORIDE (UNII: 451W47IQ8X) 8.13 ug in 10 mm SODIUM THIOSULFATE (UNII: HX1032V43M) 2.46 ug in 10 mm SODIUM PHOSPHATE, MONOBASIC (UNII: 3980JIH2SW) 6.15 ug in 10 mm Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10129-001-03 2740 mm in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/26/1987 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 06/26/1987 GINGI-PAK MAX SOFT-TWIST NO 2
dl epinephrine hci solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10129-002 Route of Administration PERIODONTAL, DENTAL, SUBGINGIVAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPINEPHRINE HYDROCHLORIDE (UNII: WBB047OO38) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 0.197 mg in 10 mm Inactive Ingredients Ingredient Name Strength COTTON FIBER (UNII: 70LDW53ROO) 10 mm in 10 mm SODIUM DITHIONITE (UNII: 2K5B8F6ES1) 6.15 ug in 10 mm EDETIC ACID (UNII: 9G34HU7RV0) 0.033 ug in 10 mm METHYLPARABEN (UNII: A2I8C7HI9T) 1.65 ug in 10 mm PROPYLPARABEN (UNII: Z8IX2SC1OH) 0.813 ug in 10 mm SODIUM CHLORIDE (UNII: 451W47IQ8X) 8.13 ug in 10 mm SODIUM THIOSULFATE (UNII: HX1032V43M) 2.46 ug in 10 mm SODIUM PHOSPHATE, MONOBASIC (UNII: 3980JIH2SW) 6.15 ug in 10 mm Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10129-002-03 2740 mm in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/11/1985 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/11/1985 GINGI-PAK MAX Z-TWIST NO 1
dl epinephrine hci solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10129-052 Route of Administration DENTAL, SUBGINGIVAL, PERIODONTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPINEPHRINE HYDROCHLORIDE (UNII: WBB047OO38) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 0.197 mg in 10 mm Inactive Ingredients Ingredient Name Strength COTTON FIBER (UNII: 70LDW53ROO) 10 mm in 10 mm SODIUM DITHIONITE (UNII: 2K5B8F6ES1) 6.15 ug in 10 mm EDETIC ACID (UNII: 9G34HU7RV0) 0.033 ug in 10 mm SODIUM THIOSULFATE (UNII: HX1032V43M) 2.46 ug in 10 mm SODIUM PHOSPHATE, MONOBASIC (UNII: 3980JIH2SW) 6.15 ug in 10 mm SODIUM CHLORIDE (UNII: 451W47IQ8X) 8.13 ug in 10 mm PROPYLPARABEN (UNII: Z8IX2SC1OH) 0.813 ug in 10 mm METHYLPARABEN (UNII: A2I8C7HI9T) 1.65 ug in 10 mm Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10129-052-01 2740 mm in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 05/24/1995 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/24/1995 GINGI-PAK MAX Z-TWIST NO 2
dl epinephrine hci solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10129-053 Route of Administration DENTAL, SUBGINGIVAL, PERIODONTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPINEPHRINE HYDROCHLORIDE (UNII: WBB047OO38) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 0.197 mg in 10 mm Inactive Ingredients Ingredient Name Strength COTTON FIBER (UNII: 70LDW53ROO) 10 mm in 10 mm SODIUM DITHIONITE (UNII: 2K5B8F6ES1) 6.15 ug in 10 mm EDETIC ACID (UNII: 9G34HU7RV0) 0.033 ug in 10 mm SODIUM THIOSULFATE (UNII: HX1032V43M) 2.46 ug in 10 mm SODIUM PHOSPHATE, MONOBASIC (UNII: 3980JIH2SW) 6.15 ug in 10 mm SODIUM CHLORIDE (UNII: 451W47IQ8X) 8.13 ug in 10 mm PROPYLPARABEN (UNII: Z8IX2SC1OH) 0.813 ug in 10 mm METHYLPARABEN (UNII: A2I8C7HI9T) 1.65 ug in 10 mm Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10129-053-01 2740 mm in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 05/24/1995 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/24/1995 GINGI-PAK MAX Z-TWIST NO 3
dl epinephrine hci solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10129-054 Route of Administration SUBGINGIVAL, DENTAL, PERIODONTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPINEPHRINE HYDROCHLORIDE (UNII: WBB047OO38) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 0.197 mg in 10 mm Inactive Ingredients Ingredient Name Strength COTTON FIBER (UNII: 70LDW53ROO) 10 mm in 10 mm SODIUM DITHIONITE (UNII: 2K5B8F6ES1) 6.15 ug in 10 mm EDETIC ACID (UNII: 9G34HU7RV0) 0.033 ug in 10 mm SODIUM THIOSULFATE (UNII: HX1032V43M) 2.46 ug in 10 mm SODIUM PHOSPHATE, MONOBASIC (UNII: 3980JIH2SW) 6.15 ug in 10 mm SODIUM CHLORIDE (UNII: 451W47IQ8X) 8.13 ug in 10 mm PROPYLPARABEN (UNII: Z8IX2SC1OH) 0.813 ug in 10 mm METHYLPARABEN (UNII: A2I8C7HI9T) 1.65 ug in 10 mm Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10129-054-01 2740 mm in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 05/24/1995 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/24/1995 GINGI-PAK MAX 2-PLY
dl epinephrine hci solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10129-004 Route of Administration PERIODONTAL, DENTAL, SUBGINGIVAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPINEPHRINE HYDROCHLORIDE (UNII: WBB047OO38) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 0.197 mg in 10 mm Inactive Ingredients Ingredient Name Strength COTTON FIBER (UNII: 70LDW53ROO) 10 mm in 10 mm SODIUM DITHIONITE (UNII: 2K5B8F6ES1) 6.15 ug in 10 mm EDETIC ACID (UNII: 9G34HU7RV0) 0.033 ug in 10 mm METHYLPARABEN (UNII: A2I8C7HI9T) 1.65 ug in 10 mm PROPYLPARABEN (UNII: Z8IX2SC1OH) 0.813 ug in 10 mm SODIUM CHLORIDE (UNII: 451W47IQ8X) 8.13 ug in 10 mm SODIUM THIOSULFATE (UNII: HX1032V43M) 2.46 ug in 10 mm SODIUM PHOSPHATE, MONOBASIC (UNII: 3980JIH2SW) 6.15 ug in 10 mm Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10129-004-03 2740 mm in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/29/1984 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/29/1984 GINGI-PAK MAX Z-TWIST NO 00
dl epinephrine hci solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10129-051 Route of Administration DENTAL, SUBGINGIVAL, PERIODONTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPINEPHRINE HYDROCHLORIDE (UNII: WBB047OO38) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 0.197 mg in 10 mm Inactive Ingredients Ingredient Name Strength COTTON FIBER (UNII: 70LDW53ROO) 10 mm in 10 mm SODIUM DITHIONITE (UNII: 2K5B8F6ES1) 6.15 ug in 10 mm EDETIC ACID (UNII: 9G34HU7RV0) 0.033 ug in 10 mm SODIUM THIOSULFATE (UNII: HX1032V43M) 2.46 ug in 10 mm SODIUM PHOSPHATE, MONOBASIC (UNII: 3980JIH2SW) 6.15 ug in 10 mm SODIUM CHLORIDE (UNII: 451W47IQ8X) 8.13 ug in 10 mm PROPYLPARABEN (UNII: Z8IX2SC1OH) 0.813 ug in 10 mm METHYLPARABEN (UNII: A2I8C7HI9T) 1.65 ug in 10 mm Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10129-051-01 2740 mm in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 05/24/1995 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/24/1995 GINGI-PAK MAX SOFT-TWIST NO 3
dl epinephrine hci solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10129-003 Route of Administration PERIODONTAL, DENTAL, SUBGINGIVAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPINEPHRINE HYDROCHLORIDE (UNII: WBB047OO38) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 0.197 mg in 10 mm Inactive Ingredients Ingredient Name Strength COTTON FIBER (UNII: 70LDW53ROO) 10 mm in 10 mm SODIUM DITHIONITE (UNII: 2K5B8F6ES1) 6.15 ug in 10 mm EDETIC ACID (UNII: 9G34HU7RV0) 0.033 ug in 10 mm METHYLPARABEN (UNII: A2I8C7HI9T) 1.65 ug in 10 mm PROPYLPARABEN (UNII: Z8IX2SC1OH) 0.813 ug in 10 mm SODIUM CHLORIDE (UNII: 451W47IQ8X) 8.13 ug in 10 mm SODIUM THIOSULFATE (UNII: HX1032V43M) 2.46 ug in 10 mm SODIUM PHOSPHATE, MONOBASIC (UNII: 3980JIH2SW) 6.15 ug in 10 mm Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10129-003-03 2740 mm in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/11/1985 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/11/1985 CROWN-PAK MAX 4-PLY
dl epinephrine hci solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10129-005 Route of Administration PERIODONTAL, DENTAL, SUBGINGIVAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPINEPHRINE HYDROCHLORIDE (UNII: WBB047OO38) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 0.197 mg in 10 mm Inactive Ingredients Ingredient Name Strength COTTON FIBER (UNII: 70LDW53ROO) 10 mm in 10 mm SODIUM DITHIONITE (UNII: 2K5B8F6ES1) 6.15 ug in 10 mm EDETIC ACID (UNII: 9G34HU7RV0) 0.033 ug in 10 mm METHYLPARABEN (UNII: A2I8C7HI9T) 1.65 ug in 10 mm PROPYLPARABEN (UNII: Z8IX2SC1OH) 0.813 ug in 10 mm SODIUM CHLORIDE (UNII: 451W47IQ8X) 8.13 ug in 10 mm SODIUM THIOSULFATE (UNII: HX1032V43M) 2.46 ug in 10 mm SODIUM PHOSPHATE, MONOBASIC (UNII: 3980JIH2SW) 6.15 ug in 10 mm Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10129-005-03 2740 mm in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/29/1984 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/29/1984 GINGI-PAK COTTON COIL
dl epinephrine hci solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10129-008 Route of Administration PERIODONTAL, DENTAL, SUBGINGIVAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPINEPHRINE HYDROCHLORIDE (UNII: WBB047OO38) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 1.18 mg in 10 mm Inactive Ingredients Ingredient Name Strength COTTON FIBER (UNII: 70LDW53ROO) 10 mm in 10 mm SODIUM DITHIONITE (UNII: 2K5B8F6ES1) 6.15 ug in 10 mm EDETIC ACID (UNII: 9G34HU7RV0) 0.033 ug in 10 mm METHYLPARABEN (UNII: A2I8C7HI9T) 1.65 ug in 10 mm PROPYLPARABEN (UNII: Z8IX2SC1OH) 0.813 ug in 10 mm SODIUM CHLORIDE (UNII: 451W47IQ8X) 8.13 ug in 10 mm SODIUM THIOSULFATE (UNII: HX1032V43M) 2.46 ug in 10 mm SODIUM PHOSPHATE, MONOBASIC (UNII: 3980JIH2SW) 6.15 ug in 10 mm Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10129-008-01 610 mm in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/26/1987 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 06/26/1987 GINGI-PAK PELLETS
dl epinephrine hci solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10129-007 Route of Administration PERIODONTAL, DENTAL, SUBGINGIVAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPINEPHRINE HYDROCHLORIDE (UNII: WBB047OO38) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 0.7 mg Inactive Ingredients Ingredient Name Strength SODIUM DITHIONITE (UNII: 2K5B8F6ES1) 2.38 ug EDETIC ACID (UNII: 9G34HU7RV0) 0.013 ug METHYLPARABEN (UNII: A2I8C7HI9T) 0.638 ug PROPYLPARABEN (UNII: Z8IX2SC1OH) 0.314 ug SODIUM CHLORIDE (UNII: 451W47IQ8X) 3.14 ug SODIUM THIOSULFATE (UNII: HX1032V43M) 0.95 ug SODIUM PHOSPHATE, MONOBASIC (UNII: 3980JIH2SW) 2.38 ug Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10129-007-02 500 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/26/1987 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 06/26/1987 Labeler - Gingi-Pak a Division of the Belport (008480121) Registrant - Jeff Nichols (008480121) Establishment Name Address ID/FEI Business Operations Gingi-Pak a Division of the Belport 008480121 manufacture(10129-051, 10129-052, 10129-053, 10129-054, 10129-001, 10129-002, 10129-003, 10129-004, 10129-005, 10129-007, 10129-008)