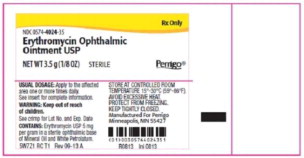
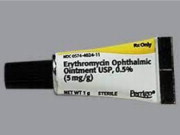
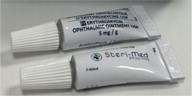
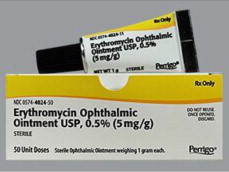
Label: ERYTHROMYCIN ointment
- NDC Code(s): 48102-057-11
- Packager: Fera Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Unapproved drug for use in drug shortage
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 26, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HEALTH CARE PROVIDER LETTER
January 9, 2024
IMPORTANT PRESCRIBING INFORMATION
Subject:
Temporary Importation of Steri-Med Pharma, Inc.'s Erythromycin Ophthalmic Ointment 5mg/g to Address Drug Shortage
Dear Healthcare Provider,
In order to address the current drug shortage of Erythromycin Ophthalmic Ointment, Fera Pharmaceuticals, LLC (Fera), in conjunction with Steri-Med Pharma, Inc. (Steri-Med), is coordinating with the U.S. FDA Drug Shortage Staff to increase its availability in the U.S. market by temporary importation of non-FDA approved product from Canada. Effective immediately and during this temporary period, Fera will distribute the following product:
This supply of Erythromycin Ophthalmic Ointment is approved and marketed in Canada, under DIN 02326663, and manufactured by Steri-Med (previously known as Sterigen), a facility licensed by Health Canada. At this time, no other entity except Steri-Med or its distributor Fera is authorized by the FDA to import or distribute Steri-Med's Erythromycin Ophthalmic Ointment in the U.S.
It is important to note the following:
- The strength and qualitative composition of the imported drug product are the same as the FDA- approved drug product. Both also meet the USP monograph specification.
-
The linear barcode on the imported product label may not register accurately on U.S. scanning systems. Institutions should manually input the product information into their systems to confirm that barcode systems do not provide incorrect information when the product is scanned. Alternative procedures should be followed to assure that the correct drug product is being used and administered to individual patients. The linear barcode is provided in the Table 1 above and Appendix 1 to assist with input of product information in the institutional setting.
In addition, the carton of the imported product does not include a product identifier as required under the Drug Supply Chain Security Act (DSCSA). Specifically, each package does not include the NDC, unique serial number, lot number, and expiration date in both human-readable and a two-dimensional data matrix barcode. Additionally, the imported product may not be accompanied with DSCSA product tracing documentation (transaction information, transaction history, and transaction statement). - The drug product being distributed by Fera Pharmaceuticals, LLC is approved in Canada. The Prescribing Information (PI) and Patient Medication Information (Medication Guide) reviewed and approved by Health Canada differ from the full prescribing information for the current U.S. FDA Reference Standard (RS). The U.S. FDA label does not contain a Medication Guide.
In particular, the FDA label provides more detailed information regarding DESCRIPTION, CLINICAL PHARMACOLOGY, USAGE during pregnancy and in infants, and in PRECAUTIONS.
Please refer to the package insert for the U.S. FDA-approved Erythromycin Ophthalmic Ointment USP, 5 mg/g for full prescribing information.
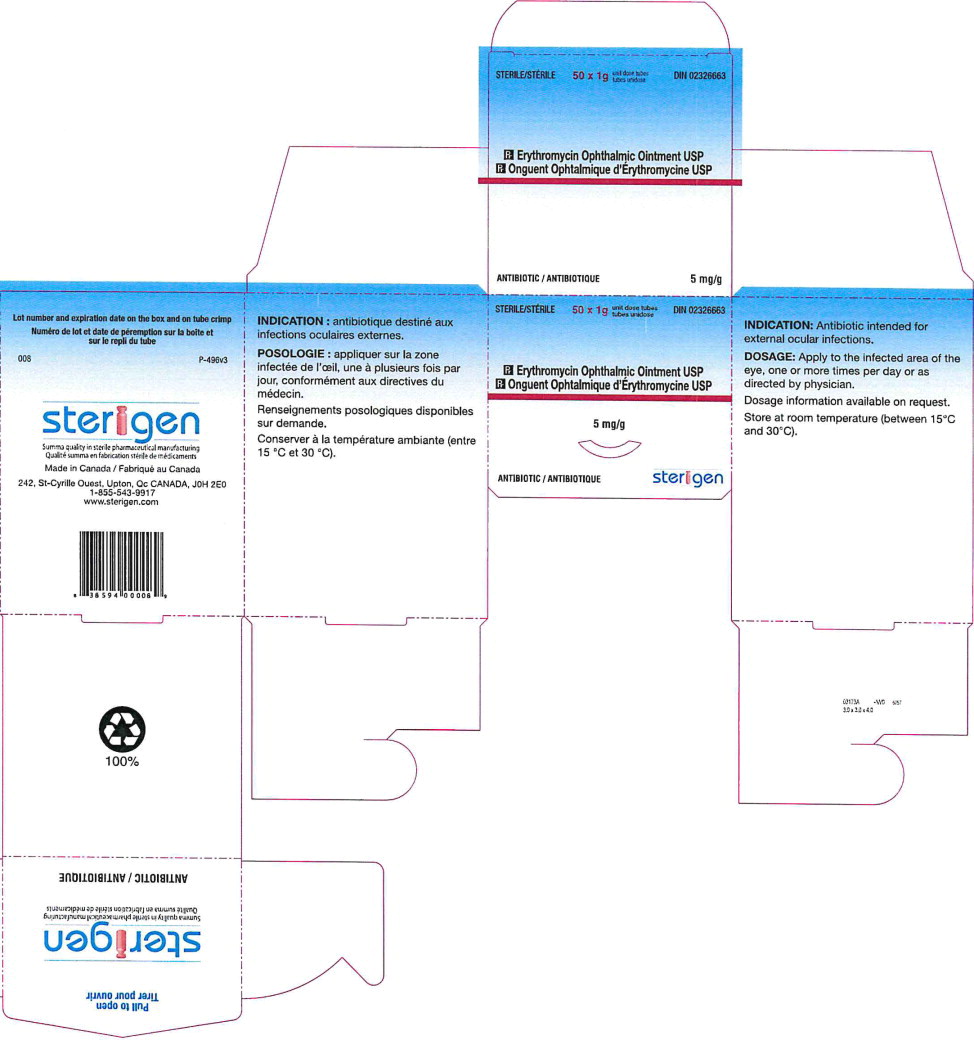
Table 2. Comparison Between the FDA-approved vs. Steri-Med Carton and Tube Labels FDA-approved Product Labels Imported Product Labels from Steri-Med (formerly Sterigen)* Tube Carton To order, please call (414) 434-6604. For additional questions about the information contained in this letter, please contact Fera at (516) 277-1449.
Healthcare providers and patients are encouraged to report adverse events in patients using imported Erythromycin Ophthalmic Ointment to Fera at (414) 434-6604.
Adverse events or quality problems experienced with the use of this product may also be reported to the FDA's MedWatch Adverse Event Reporting Program either online, by regular mail, or by fax:
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form or submit by fax to 1-800-FDA-0178 (1-800-332-0178).
Sincerely,
Michelle Kim, PharmD.
Sr. Director of Regulatory Affairs
- Principal Display Panel - 5 mg Carton Label (Steri-Med)
- Principal Display Panel - 5 mg Carton Label (Sterigen)
-
INGREDIENTS AND APPEARANCE
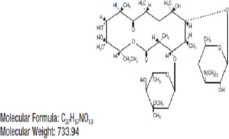
ERYTHROMYCIN
erythromycin ointmentProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:48102-057 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ERYTHROMYCIN (UNII: 63937KV33D) (ERYTHROMYCIN - UNII:63937KV33D) ERYTHROMYCIN 5 mg in 1 g Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) PETROLATUM (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:48102-057-11 50 in 1 CARTON 09/27/2023 1 1 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 09/27/2023 Labeler - Fera Pharmaceuticals, LLC (831023713)