Label: ANTI-ITCH EXTRA STRENGTH- diphenhydramine hydrochloride, zinc acetate cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 29500-2437-1 - Packager: Personal Care Products, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 11, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
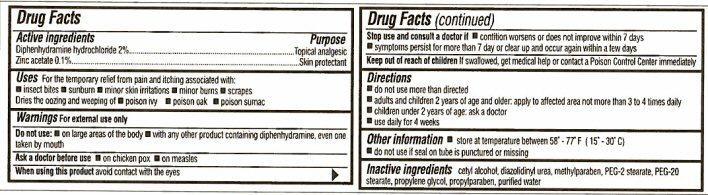
- Active ingredients
- Purpose
- Keep out of reach of children
- Uses
-
Warnings
For external use only
Do not use:
- on large areas of the body
- with any other product containing diphenhydramine, even one taken by mouth
Ask a doctor before use
- on chicken pox
- on measles
When using this product avoid contact with the eyes
Stop use and ask a doctor if
- condition worsens or does not improve within 7 days
- symptoms persist for more than 7 day or clear up and occur again within a few days
- Directions
- Other information
- Inactive ingredients
-
Product Label
DRUGSTORE-Rx
SMART WELLNESS
Extra Strength
Anti-Itch Cream
Topical Analgesic and Skin Protectant
Histamine Blocker
Relieves itching from
* Poison ivy
* Poison sumac
* Sunburn
* Insect bites
NET WT. 1.25 OZ. (35 g)
DRUGSTORE-Rx
SMART WELLNESS delivers outstanding quality in healthcare products, at truly amazing prices. We employ the highest standard when searching the world to bring the best value to your doorstep. Use our products confidently, knowing that attention and care has gone into every item. That's smart wellness!
Distributed by: PERSONAL CARE PRODUCTS, LLC
Troy, Michigan 48084 U.S.A.
Code No. MH/DRUGS/KD-313 Made in India

-
INGREDIENTS AND APPEARANCE
ANTI-ITCH EXTRA STRENGTH
diphenhydramine hydrochloride, zinc acetate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:29500-2437 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 700 mg in 35 g ZINC ACETATE (UNII: FM5526K07A) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 35 mg in 35 g Inactive Ingredients Ingredient Name Strength CETYL ALCOHOL (UNII: 936JST6JCN) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) METHYLPARABEN (UNII: A2I8C7HI9T) PEG-2 STEARATE (UNII: 94YQ11Y95F) PEG-20 STEARATE (UNII: NBX892EA57) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:29500-2437-1 35 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 07/10/2013 Labeler - Personal Care Products, Inc. (966155082) Registrant - Personal Care Products, Inc. (966155082) Establishment Name Address ID/FEI Business Operations Anicare Pharmaceuticals Pvt. Ltd. 916837425 manufacture(29500-2437)


