Label: CARDURA- doxazosin mesylate tablet
CARDURA- doxazosin tablet
-
NDC Code(s):
0049-2410-10,
0049-2512-10,
0049-2614-10,
0049-2716-10, view more0049-2750-41, 0049-2750-66, 0049-2760-41, 0049-2760-66, 0049-2770-41, 0049-2770-66, 0049-2780-41, 0049-2780-66
- Packager: ROERIG
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 20, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CARDURA safely and effectively. See full prescribing information for CARDURA.
CARDURA ® (doxazosin) tablets, for oral use
Initial U.S. Approval: 1990INDICATIONS AND USAGE
CARDURA is an alpha1 adrenergic antagonist indicated for: (1)
- •
- Signs and symptoms of Benign Prostatic Hyperplasia (BPH)
- •
- Treatment of Hypertension
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
- •
- Tablets: 1 mg, 2 mg, 4 mg, 8 mg.
CONTRAINDICATIONS
- •
- Hypersensitivity to doxazosin, other quinazolines, or any other ingredient in CARDURA. (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
The most commonly reported adverse reactions from clinical trials are Fatigue, malaise, hypotension, and dizziness. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Pfizer, Inc. at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 1/2022
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Benign Prostatic Hyperplasia (BPH)
1.2 Hypertension
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Information
2.2 Benign Prostatic Hyperplasia
2.3 Hypertension
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Postural Hypotension
5.2 Cataract Surgery
5.3 Prostate Cancer
5.4 Priapism
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1. CYP 3A Inhibitors
7.2 Phosphodiesterase-5 (PDE-5) inhibitors
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and Pharmacology
14 CLINICAL STUDIES
14.1 Benign Prostatic Hyperplasia (BPH)
14.2 Hypertension
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Benign Prostatic Hyperplasia (BPH)
CARDURA is indicated for the treatment of the signs and symptoms of BPH.
1.2 Hypertension
CARDURA is indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes, including this drug.
Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than one drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program's Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC).
Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly.
Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal.
Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy.
CARDURA may be used alone or in combination with other antihypertensives.
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Information
Following the initial dose and with each dose increase of CARDURA, monitor blood pressure for at least 6 hours following administration. If CARDURA administration is discontinued for several days, therapy should be restarted using the initial dosing regimen.
2.2 Benign Prostatic Hyperplasia
The recommended initial dosage of CARDURA is 1 mg given once daily either in the morning or evening.
Depending on the individual patient's urodynamics and BPH symptomatology, the dose may be titrated at 1 to 2 week intervals to 2 mg, and thereafter to 4 mg and 8 mg once daily. The maximum recommended dose for BPH is 8 mg once daily.
Routinely monitor blood pressure in these patients.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Postural Hypotension
Postural hypotension with or without symptoms (e.g., dizziness) may develop within a few hours following administration of CARDURA. However, infrequently, symptomatic postural hypotension has also been reported later than a few hours after dosing. As with other alpha-blockers, there is a potential for syncope, especially after the initial dose or after an increase in dosage strength. Advise patient how to avoid symptoms resulting from postural hypotension and what measures to take should they develop.
Concomitant administration of CARDURA with a PDE-5 inhibitor can result in additive blood pressure lowering effects and symptomatic hypotension.
5.2 Cataract Surgery
Intraoperative Floppy Iris Syndrome (IFIS) has been observed during cataract surgery in some patients on or previously treated with alpha1 blockers. This variant of small pupil syndrome is characterized by the combination of a flaccid iris that billows in response to intraoperative irrigation currents, progressive intraoperative miosis despite preoperative dilation with standard mydriatic drugs, and potential prolapse of the iris toward the phacoemulsification incisions. The patient's surgeon should be prepared for possible modifications to their surgical technique, such as the utilization of iris hooks, iris dilator rings, or viscoelastic substances. There does not appear to be a benefit of stopping alpha1 blocker therapy prior to cataract surgery.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Benign Prostatic Hyperplasia (BPH)
The incidence of adverse events has been ascertained from worldwide clinical trials in 965 BPH patients. The incidence rates presented below (Table 2) are based on combined data from seven placebo-controlled trials involving once-daily administration of CARDURA in doses of 1 to 16 mg in hypertensives and 0.5 to 8 mg in normotensives. Adverse reactions occurring more than 1% more frequently in BPH patients treated with CARDURA vs placebo are summarized in Table 1.
Table 1. Adverse Reactions Occurring more than 1% More Frequently in BPH Patients Treated with Cardura Versus Placebo BODY SYSTEM Cardura
N=665Placebo
N=300- *
- Includes vertigo
NERVOUS SYSTEM DISORDERS
Dizziness*
15.6%
9.0%
Somnolence
3.0%
1.0%
CARDIAC DISORDERS
Hypotension
1.7%
0%
RESPIRATORY, THORACIC AND MEDIASTINAL DISORDERS
Dyspnoea
2.6%
0.3%
GASTROINTESTINAL DISORDERS
Dry Mouth
1.4%
0.3%
GENERAL DISORDERS AND ADMINISTRATION SITE CONDITIONS
Fatigue
8.0%
1.7%
Oedema
2.7%
0.7%
Other adverse reactions occurring less than 1% more frequently in BPH patients treated with CARDURA vs placebo but plausibly related to CARDURA include: palpitations.
Hypertension
CARDURA has been administered to approximately 4000 hypertensive patients in clinical trials, of whom 1679 were included in the hypertension clinical development program. In placebo-controlled studies, adverse events occurred in 49% and 40% of patients in the doxazosin and placebo groups, respectively, and led to discontinuation in 2% of patients in each group.
Adverse reactions occurring more than 1% more frequently in hypertensive patients treated with CARDURA vs placebo are summarized in Table 1. . Postural effects and edema appeared to be dose-related. The prevalence rates presented below are based on combined data from placebo-controlled studies involving once-daily administration of doxazosin at doses ranging from 1 to 16 mg.
Table 2. Adverse Reactions Occurring more than 1% More Frequently in Hypertensive Patients Treated with Cardura versus Placebo BODY SYSTEM Cardura
N=339Placebo
N=336NERVOUS SYSTEM DISORDERS
Dizziness
19%
9%
Somnolence
5%
1%
RESPIRATORY, THORACIC AND MEDIASTINAL DISORDERS
Rhinitis
3%
1%
RENAL AND URINARY DISORDERS
Polyuria
2%
0%
REPRODUCTIVE SYSTEM AND BREAST DISORDERS GENERAL DISORDERS AND ADMINISTRATION SITE CONDITIONS
Fatigue / Malaise
12%
6%
Other adverse reactions occurring less than 1% more frequently in hypertensive patients treated with CARDURA vs placebo but plausibly related to CARDURA use include vertigo, hypotension, hot flushes, epistaxis and oedema.
CARDURA has been associated with decreases in white blood cell counts
Laboratory changes observed in clinical studies
Leukopenia/Neutropenia: Decreases in mean white blood cell (WBC) and mean neutrophil count were observed in controlled clinical trials of hypertensive patients receiving CARDURA. In cases where follow-up was available, WBC and neutrophil counts returned to normal after discontinuation of CARDURA. No patients became symptomatic as a result of the low WBC or neutrophil counts.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of CARDURA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
In post-marketing experience, the following additional adverse reactions have been reported:
Blood and Lymphatic System Disorders: leukopenia, thrombocytopenia;
Immune System Disorders: allergic reaction;
Nervous System Disorders: hypoesthesia;
Eye Disorders: Intraoperative Floppy Iris Syndrome [see Warnings and precautions (5.4)];
Cardiac Disorders: bradycardia;
Respiratory, Thoracic and Mediastinal Disorders: bronchospasm aggravated;
Gastrointestinal Disorders: vomiting;
Hepatobiliary Disorders: cholestasis, hepatitis cholestatic;
Skin and Subcutaneous Tissue Disorders: urticaria;
Musculoskeletal and Connective Tissue Disorders: muscle cramps, muscle weakness;
Renal and Urinary Disorders: hematuria, micturition disorder, micturition frequency, nocturia;
Reproductive System and Breast Disorders: gynecomastia, priapism. -
7 DRUG INTERACTIONS
7.1. CYP 3A Inhibitors
In vitro studies suggest that doxazosin is a substrate of CYP 3A4. Strong CYP3A inhibitors may increase exposure to doxazosin. Monitor blood pressure and for symptoms of hypotension when CARDURA is used concomitantly with strong CYP3A inhibitors [see Clinical Pharmacology (12.3)].
7.2 Phosphodiesterase-5 (PDE-5) inhibitors
Concomitant administration of CARDURA with a phosphodiesterase-5 (PDE-5) inhibitor can result in additive blood pressure lowering effects and symptomatic hypotension. Monitor blood pressure and for symptoms of hypotension [see Warnings and Precautions (5.1)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The limited available data with CARDURA in pregnant women are not sufficient to inform a drug-associated risk for major birth defects and miscarriage. However, untreated hypertension during pregnancy can result in increased maternal risks [see Clinical Considerations]. In animal reproduction studies, no adverse developmental effects were observed when doxazosin was orally administered to pregnant rabbits and rats during the period of organogenesis at doses of up to 41 and 20 mg/kg, respectively (exposures in rabbits and rats were 10 and 4 times, respectively, the human AUC exposures with a 12 mg/day therapeutic dose). A dosage regimen of 82 mg/kg/day in the rabbit was associated with reduced fetal survival [see Data].
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2–4% and 15–20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Hypertension in pregnancy increases the maternal risk for pre-eclampsia, gestational diabetes, premature delivery, and delivery complications (e.g., need for cesarean section, and post-partum hemorrhage). Hypertension increases the fetal risk for intrauterine growth restriction and intrauterine death.
Data
Animal Data
Radioactivity was found to cross the placenta following oral administration of labelled doxazosin to pregnant rats. Studies in pregnant rabbits and rats at daily oral doses of up to 41 and 20 mg/kg, respectively (plasma drug concentrations of 10 and 4 times, respectively, the human AUC exposures with a 12 mg/day therapeutic dose), have revealed no evidence of adverse developmental effects. A dosage regimen of 82 mg/kg/day in the rabbit was associated with reduced fetal survival. In peri- and postnatal studies in rats, postnatal development at maternal doses of 40 or 50 mg/kg/day of doxazosin (about 8 times human AUC exposure with a 12 mg/day therapeutic dose) was delayed, as evidenced by slower body weight gain and slightly later appearance of anatomical features and reflexes.
8.2 Lactation
Risk Summary
There is limited information on the presence of CARDURA in human milk [see Data]. There is no information on the effects of CARDURA on the breastfeed infant or the effects on milk production.
8.5 Geriatric Use
Benign Prostatic Hyperplasia (BPH)
The safety and effectiveness profile of CARDURA was similar in the elderly (age ≥ 65 years) and younger (age < 65 years) patients.
Hypertension
Clinical studies of CARDURA did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
8.6 Hepatic Impairment
CARDURA is extensively metabolized in the liver. Hepatic impairment is expected to increase exposure to doxazosin. Use of CARDURA in patients with severe hepatic impairment (Child-Pugh Class C) is not recommended. Monitor blood pressure and for symptoms of hypotension in patients with lesser degrees of hepatic impairment (Child-Pugh Class A and B) [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
Experience with CARDURA overdosage is limited. Two adolescents, who each intentionally ingested 40 mg CARDURA with diclofenac or acetaminophen, were treated with gastric lavage with activated charcoal and made full recoveries. A two-year-old child who accidently ingested 4 mg CARDURA was treated with gastric lavage and remained normotensive during the five-hour emergency room observation period. A six-month-old child accidentally received a crushed 1 mg tablet of CARDURA and was reported to have been drowsy. A 32-year-old female with chronic renal failure, epilepsy, and depression intentionally ingested 60 mg CARDURA (blood level = 0.9 mcg/mL; normal values in hypertensives = 0.02 mcg/mL); death was attributed to a grand mal seizure resulting from hypotension. A 39-year-old female who ingested 70 mg CARDURA, alcohol, and Dalmane® (flurazepam) developed hypotension which responded to fluid therapy.
The oral LD50 of doxazosin is greater than 1000 mg/kg in mice and rats. The most likely manifestation of overdosage would be hypotension, for which the usual treatment would be intravenous infusion of fluid. As doxazosin is highly protein bound, dialysis would not be indicated.
-
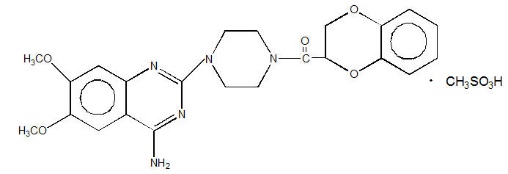
11 DESCRIPTION
CARDURA® (doxazosin) is a quinazoline compound that is a selective inhibitor of the alpha1 subtype of alpha-adrenergic receptors. The chemical name of doxazosin mesylate is 1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-(1,4-benzodioxan-2-ylcarbonyl) piperazine methanesulfonate. The empirical formula for doxazosin mesylate is C23H25N5O5 ∙ CH4O3S and the molecular weight is 547.6. It has the following structure:
CARDURA (doxazosin) is freely soluble in dimethylsulfoxide, soluble in dimethylformamide, slightly soluble in methanol, ethanol, and water (0.8% at 25°C), and very slightly soluble in acetone and methylene chloride. CARDURA is available as colored tablets for oral use and contains doxazosin mesylate equivalent to 1 mg (white), 2 mg (yellow or white), 4 mg (orange or white) and 8 mg (green or white) of doxazosin as the free base.
The inactive ingredients for all tablets are microcrystalline cellulose, lactose, sodium starch glycolate, magnesium stearate and sodium lauryl sulfate. The 2 mg yellow tablet contains D & C yellow 10 and FD & C yellow 6; the 4 mg orange tablet contains FD & C yellow 6; the 8 mg green tablet contains FD & C blue 2 and D & C yellow 10.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Benign Prostatic Hyperplasia (BPH)
The symptoms associated with benign prostatic hyperplasia (BPH), such as urinary frequency, nocturia, weak stream, hesitancy, and incomplete emptying are related to two components, anatomical (static) and functional (dynamic). The static component is related to an increase in prostate size caused, in part, by a proliferation of smooth muscle cells in the prostatic stroma. However, the severity of BPH symptoms and the degree of urethral obstruction do not correlate well with the size of the prostate. The dynamic component of BPH is associated with an increase in smooth muscle tone in the prostate and bladder neck. The degree of tone in this area is mediated by the alpha1 adrenoceptor, which is present in high density in the prostatic stroma, prostatic capsule and bladder neck. Blockade of the alpha1 receptor decreases urethral resistance and may relieve the obstruction and BPH symptoms and improve urine flow.
Hypertension
The mechanism of action of CARDURA is selective blockade of the alpha1 (postjunctional) subtype of adrenergic receptors. Studies in normal human subjects have shown that doxazosin competitively antagonized the pressor effects of phenylephrine (an alpha1 agonist) and the systolic pressor effect of norepinephrine. Doxazosin and prazosin have similar abilities to antagonize phenylephrine. The antihypertensive effect of CARDURA results from a decrease in systemic vascular resistance. The parent compound doxazosin is primarily responsible for the antihypertensive activity. The low plasma concentrations of known active and inactive metabolites of doxazosin (2-piperazinyl, 6'- and 7'-hydroxy and 6- and 7-O-desmethyl compounds) compared to parent drug indicate that the contribution of even the most potent compound (6'-hydroxy) to the antihypertensive effect of doxazosin in man is probably small. The 6'- and 7'-hydroxy metabolites have demonstrated antioxidant properties at concentrations of 5 µM, in vitro.
12.2 Pharmacodynamics
Benign Prostatic Hyperplasia (BPH)
Administration of CARDURA to patients with symptomatic BPH resulted in a statistically significant improvement in maximum urinary flow rate [see Clinical Studies (14.1)].
Effect on Normotensive Patients with Benign Prostatic Hyperplasia (BPH)
Although blockade of alpha1 adrenoceptors also lowers blood pressure in hypertensive patients with increased peripheral vascular resistance, CARDURA treatment of normotensive men with BPH did not result in a clinically significant blood pressure lowering effect (Table 4). The proportion of normotensive patients with a sitting systolic blood pressure less than 90 mmHg and/or diastolic blood pressure less than 60 mmHg at any time during treatment with CARDURA 1–8 mg once daily was 6.7% with doxazosin and not significantly different (statistically) from that with placebo (5%).
Hypertension
Administration of CARDURA results in a reduction in systemic vascular resistance. In patients with hypertension, there is little change in cardiac output. Maximum reductions in blood pressure usually occur 2–6 hours after dosing and are associated with a small increase in standing heart rate. Like other alpha1-adrenergic blocking agents, doxazosin has a greater effect on blood pressure and heart rate in the standing position.
12.3 Pharmacokinetics
Absorption
After oral administration of therapeutic doses, peak plasma levels of CARDURA occur at about 2–3 hours. Bioavailability is approximately 65%, reflecting first-pass metabolism of doxazosin by the liver. The effect of food on the pharmacokinetics of CARDURA was examined in a crossover study with twelve hypertensive subjects. Reductions of 18% in mean maximum plasma concentration (Cmax) and 12% in the area under the concentration-time curve (AUC) occurred when CARDURA was administered with food. Neither of these differences is clinically significant.
In a crossover study in 24 normotensive subjects, the pharmacokinetics and safety of doxazosin were shown to be similar with morning and evening dosing regimens. The AUC after morning dosing was, however, 11% less than that after evening dosing and the time to peak concentration after evening dosing occurred significantly later than that after morning dosing (5.6 vs. 3.5 hours).
Distribution
At the plasma concentrations achieved by therapeutic doses, approximately 98% of the circulating drug is bound to plasma proteins.
Metabolism
CARDURA is extensively metabolized in the liver, mainly by O-demethylation of the quinazoline nucleus or hydroxylation of the benzodioxan moiety. In vitro studies suggest that the primary pathway for elimination is via CYP 3A4; however, CYP 2D6 and CYP 2C9 metabolic pathways are also involved to a lesser extent. Although several active metabolites of doxazosin have been identified, the pharmacokinetics of these metabolites have not been characterized.
Excretion
Plasma elimination of doxazosin is biphasic, with a terminal elimination half-life of about 22 hours. Steady-state studies in hypertensive patients given doxazosin doses of 2 to 16 mg once daily showed linear kinetics and dose proportionality. In two studies, following the administration of 2 mg orally once daily, the mean accumulation ratios (steady-state AUC vs. first-dose AUC) were 1.2 and 1.7. Enterohepatic recycling is suggested by secondary peaking of plasma doxazosin concentrations.
In a study of two subjects administered radiolabelled doxazosin 2 mg orally and 1 mg intravenously on two separate occasions, approximately 63% of the dose was eliminated in the feces and 9% of the dose was found in the urine. On average only 4.8% of the dose was excreted as unchanged drug in the feces and only a trace of the total radioactivity in the urine was attributed to unchanged drug.
Specific Populations
Geriatric
The pharmacokinetics of CARDURA in young (<65 years) and elderly (≥65 years) subjects were similar for plasma half-life values and oral clearance.
Renal Impairment
Pharmacokinetic studies in elderly patients and patients with renal impairment have shown no significant alterations compared to younger patients with normal renal function.
Hepatic Impairment
Administration of a single 2 mg dose to patients with cirrhosis (Child-Pugh Class A) showed a 40% increase in exposure to doxazosin. The impact of moderate (Child-Pugh Class B) or severe (Child-Pugh Class C) hepatic impairment on the pharmacokinetics of doxazosin is not known [see Use in Specific Populations (8.6)].
Drug Interactions
There are only limited data on the effects of drugs known to influence the hepatic metabolism of doxazosin (e.g., cimetidine).
Cimetidine: In healthy volunteers, the administration of a single 1 mg dose of doxazosin on day 1 of a four-day regimen of oral cimetidine (400 mg twice daily) resulted in a 10% increase in mean AUC of doxazosin, and a slight but not significant increase in mean Cmax and mean half-life of doxazosin.
In vitro data in human plasma indicate that CARDURA has no effect on protein binding of digoxin, warfarin, phenytoin, or indomethacin.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis and Mutagenesis: Chronic dietary administration (up to 24 months) of doxazosin mesylate at maximally tolerated doses of 40 mg/kg/day in rats and 120 mg/kg/day in mice revealed no evidence of carcinogenic potential. The highest doses evaluated in the rat and mouse studies are associated with AUCs (a measure of systemic exposure) that are 8 times and 4 times, respectively, the human AUC at a dose of 16 mg/day.
Mutagenicity studies revealed no drug- or metabolite-related effects at either chromosomal or subchromosomal levels.
Fertility in Males: Studies in rats showed reduced fertility in males treated with doxazosin at oral doses of 20 (but not 5 or 10) mg/kg/day, about 4 times the AUC exposures obtained with a 12 mg/day human dose. This effect was reversible within two weeks of drug withdrawal. There have been no reports of any effects of doxazosin on male fertility in humans.
13.2 Animal Toxicology and Pharmacology
An increased incidence of myocardial necrosis or fibrosis was observed in long-term (6–12 months) studies in rats and mice (exposure 8 times human AUC exposure in rats and somewhat equivalent to human Cmax exposure in mice). Findings were not seen at lower doses. In dogs no cardiotoxicity was observed following 12 months of oral dosing at doses that resulted in maximum plasma concentrations (Cmax) 14 times the Cmax exposure in humans receiving a 12 mg/day therapeutic dose or in Wistar rats at Cmax exposures 15 times human Cmax exposure. There is no evidence that similar lesions occur in humans.
-
14 CLINICAL STUDIES
14.1 Benign Prostatic Hyperplasia (BPH)
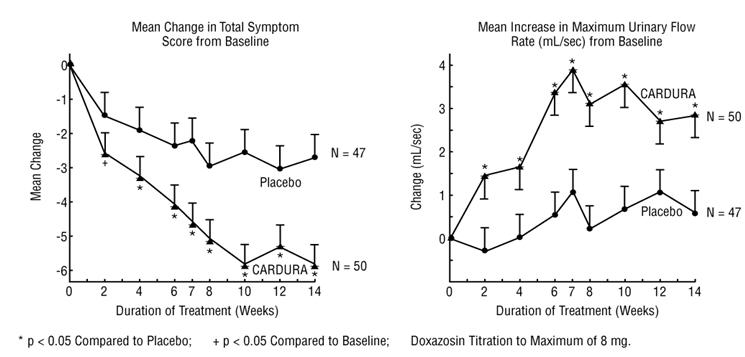
The efficacy of CARDURA was evaluated extensively in over 900 patients with BPH in double-blind, placebo-controlled trials. CARDURA treatment was superior to placebo in improving patient symptoms and urinary flow rate. Significant relief with CARDURA was seen as early as one week into the treatment regimen, with CARDURA-treated patients (N=173) showing a significant (p<0.01) increase in maximum flow rate of 0.8 mL/sec compared to a decrease of 0.5 mL/sec in the placebo group (N=41). In long-term studies, improvement was maintained for up to 2 years of treatment. In 66–71% of patients, improvements above baseline were seen in both symptoms and maximum urinary flow rate.
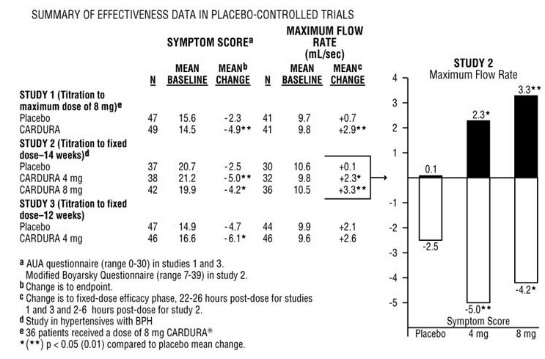
In three placebo-controlled studies of 14–16 weeks' duration, obstructive symptoms (hesitation, intermittency, dribbling, weak urinary stream, incomplete emptying of the bladder) and irritative symptoms (nocturia, daytime frequency, urgency, burning) of BPH were evaluated at each visit by patient-assessed symptom questionnaires. The bothersomeness of symptoms was measured with a modified Boyarsky questionnaire. Symptom severity/frequency was assessed using a modified Boyarsky questionnaire or an AUA-based questionnaire. Uroflowmetric evaluations were performed at times of peak (2–6 hours post-dose) and/or trough (24 hours post-dose) plasma concentrations of CARDURA.
The results from the three placebo-controlled studies (N=609) showing significant efficacy with 4 mg and 8 mg doxazosin are summarized in Table 3. In all three studies, CARDURA resulted in statistically significant relief of obstructive and irritative symptoms compared to placebo. Statistically significant improvements of 2.3–3.3 mL/sec in maximum flow rate were seen with CARDURA in Studies 1 and 2, compared to 0.1–0.7 mL/sec with placebo.
In one fixed-dose study (Study 2), CARDURA therapy (4 to 8 mg, once daily) resulted in a significant and sustained improvement in maximum urinary flow rate of 2.3–3.3 mL/sec (Table 3) compared to placebo (0.1 mL/sec). In this study, the only study in which weekly evaluations were made, significant improvement with CARDURA vs. placebo was seen after one week. The proportion of patients who responded with a maximum flow rate improvement of ≥3 mL/sec was significantly larger with CARDURA (34–42%) than placebo (13–17%). A significantly greater improvement was also seen in average flow rate with CARDURA (1.6 mL/sec) than with placebo (0.2 mL/sec). The onset and time course of symptom relief and increased urinary flow from Study 1 are illustrated in Figure 1.
14.2 Hypertension
In a pooled analysis of placebo-controlled hypertension studies with about 300 hypertensive patients per treatment group, doxazosin, at doses of 1 to 16 mg given once daily, lowered blood pressure at 24 hours by about 10/8 mmHg compared to placebo in the standing position and about 9/5 mmHg in the supine position. Peak blood pressure effects (1–6 hours) were larger by about 50–75% (i.e., trough values were about 55–70% of peak effect), with the larger peak-trough differences seen in systolic pressures. There was no apparent difference in the blood pressure response of Caucasians and blacks or of patients above and below age 65. In the same patient population, patients receiving CARDURA gained a mean of 0.6 kg compared to a mean loss of 0.1 kg for placebo patients.
TABLE 4 Mean Changes in Blood Pressure from Baseline to the Mean of the Final Efficacy Phase in Normotensives (Diastolic BP <90 mmHg) in Two Double-blind, Placebo-controlled U.S. Studies with CARDURA 1 to 8 mg once daily. PLACEBO (N=85) CARDURA (N=183) - *
- p ≤0.05 compared to placebo
Sitting BP (mmHg)
Baseline
Change
Baseline
Change
Systolic
128.4
–1.4
128.8
–4.9*
Diastolic
79.2
–1.2
79.6
–2.4*
Standing BP (mmHg)
Baseline
Change
Baseline
Change
Systolic
128.5
–0.6
128.5
–5.3*
Diastolic
80.5
–0.7
80.4
–2.6*
-
16 HOW SUPPLIED/STORAGE AND HANDLING
CARDURA (doxazosin) is available as tablets for oral administration. Each tablet contains doxazosin mesylate equivalent to 1 mg (white), 2 mg (yellow or white), 4 mg (orange or white) or 8 mg (green or white) of doxazosin as the free base.
NDC and Pack Size Strength Description NDC 0049-2750-66 (Bottle of 100)
NDC 0049-2750-41 (Unit dose of 100)1 mg
White, capsule shaped tablet engraved "Cardura" on one side, scored and engraved "1 mg" on the other side.
NDC 0049-2410-10 (Bottle of 100)
1 mg
White, round tablet engraved "CN1" on one side and "Pfizer" on the other side.
NDC 0049-2760-66 (Bottle of 100)
NDC 0049-2760-41 (Unit dose of 100)2 mg
Yellow, capsule shaped tablet engraved "Cardura" on one side, scored and engraved "2 mg" on the other side.
NDC 0049-2512-10 (Bottle of 100)
2 mg
White capsule shaped tablet with break score and engraved "CN2" on one side and "Pfizer" on the other side.
NDC 0049-2770-66 (Bottle of 100)
NDC 0049-2770-41 (Unit Dose of 100)4 mg
Orange, capsule shaped tablet engraved "Cardura" on one side, scored and engraved "4 mg" on the other side.
NDC 0049-2614-10 (Bottle of 100)
4 mg
White, diamond shaped tablet with break score and engraved "CN4" on one side and "Pfizer" on the other side.
NDC 0049-2780-66 (Bottle of 100)
NDC 0049-2780-41 (Unit dose of 100)8 mg
Green, capsule shaped tablet engraved "Cardura" on one side, scored and engraved "8 mg" on the other side.
NDC 0049-2716-10 (Bottle of 100)
8 mg
White, capsule shaped tablet with break score and engraved "CN8" on one side and "Pfizer" on the other side.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Postural Hypotension
Advise patients of the possibility of syncopal and orthostatic symptoms, especially at the initiation of therapy, and urged to avoid driving or hazardous tasks for 24 hours after the first dose, after a dosage increase, and after interruption of therapy when treatment is resumed. Advise patients to report symptoms to their healthcare provider.
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration Revised: 12/2020 PATIENT INFORMATION
CARDURA®(kar-DUR-a)
(doxazosin tablets)What is CARDURA?
- CARDURA is a prescription medicine that contains doxazosin mesylate and is called an "alpha-blocker". CARDURA is used to treat:
- •
- the symptoms of benign prostatic hyperplasia (BPH)
- •
- high blood pressure (hypertension)
It is not known if CARDURA is safe and effective in children.
Who should not take CARDURA?
- Do not take CARDURA if you:
- •
- are allergic to doxazosin, other quinazolines, or any of the ingredients in CARDURA. See the end of this Patient Information leaflet for a complete list of ingredients in CARDURA.
What should I tell my healthcare provider before taking CARDURA?
- Before taking CARDURA, tell your healthcare provider about all of your medical conditions, including if you:
- •
- have had low blood pressure, especially after taking other medicine. Signs of low blood pressure include fainting, dizziness, and lightheadedness.
- •
- have any planned eye surgery
- •
- have prostate cancer or a history of prostate cancer. Your healthcare provider may have you checked for prostate cancer before you start taking and while you take CARDURA.
- •
- have liver problems
- •
- are pregnant or plan to become pregnant. It is not known if CARDURA will harm your unborn baby.
- •
- are breastfeeding or plan to breastfeed. It is not known if CARDURA passes into your breastmilk. Talk to your healthcare provider about the best way to feed your baby if you take CARDURA.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. CARDURA may affect the way other medicines work, and other medicines may affect the way CARDURA works causing side effects.
Especially tell your healthcare provider if you take:
- •
- other medicine for high blood pressure or medicine to treat erectile dysfunction (ED) called a phosphodiesterase type 5 (PDE-5) inhibitor. The use of CARDURA with PDE-5 inhibitors can lead to a drop in blood pressure or to fainting.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take CARDURA?
- •
- Take CARDURA exactly as your healthcare provider tells you to take it.
- •
- Your healthcare provider will tell you how much CARDURA to take and when to take it.
- •
- Your healthcare provider may need to change your dose of CARDURA until it is the right dose for you.
What should I avoid while taking CARDURA?
- Do not drive or perform any hazardous task until at least 24 hours after you have taken CARDURA if you are taking:
- •
- your first dose of CARDURA
- •
- CARDURA for the first time after your healthcare provider has increased your dose of CARDURA
- •
- CARDURA for the first time after any breaks (interruptions) in your treatment with CARDURA
What are the possible side effects of CARDURA?
- CARDURA may cause serious side effects, including:
- •
- A sudden drop in blood pressure, especially when you first start treatment or when there is an increase in your dose of CARDURA, is common but can also be serious. This may cause you to faint, or to feel dizzy or lightheaded. Your risk of having this problem may be increased if you take CARDURA with certain other medicines that lower blood pressure including PDE-5 inhibitors. Your healthcare provider may monitor your blood pressure while you take CARDURA. See "What should I avoid while taking CARDURA?"
- •
- Eye problems during cataract surgery. A condition called Intraoperative Floppy Iris Syndrome (IFIS) can happen during cataract surgery if you take or have taken alpha-blockers such as CARDURA. If you need to have cataract surgery, be sure to tell your healthcare provider if you take or have taken CARDURA.
- •
- A painful erection that will not go away. CARDURA can cause a painful erection (priapism), which cannot be relieved by having sex. If this happens, get medical help right away. If priapism is not treated, you may not be able to get an erection in the future.
The most common side effects of CARDURA are:
- •
- weakness or lack of energy (asthenia)
- •
- dizziness
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of CARDURA. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of CARDURA.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use CARDURA for a condition for which it was not prescribed. Do not give CARDURA to other people, even if they have the same symptoms you have. It may harm them.
This Patient Information leaflet summarizes the most important information about CARDURA. For more information, ask your healthcare provider. You can ask your healthcare provider or pharmacist for information that is written for healthcare professionals.
What are the ingredients in CARDURA?
The size, shape and appearance of the tablet that you receive may vary, all the tablets have the same active ingredient, and this will not affect the way that the medicine works. You can identify the tablet that you have from the following information.
Strength
Either
Or
1 mg Tablet
White, round tablet marked "CN1" on one side and "Pfizer" on the other side.
White, capsule shaped tablet marked "Cardura" on one side, scored and marked "1 mg" on the other side.
2 mg Tablet
White, capsule shaped tablet with break score and marked "CN2" on one side and "Pfizer" on the other side.
Yellow, capsule shaped tablet marked "Cardura" on one side, scored and marked "2 mg" on the other side.
4 mg Tablet
White, diamond shaped tablet with break score and marked "CN4" on one side and "Pfizer" on the other side.
Orange, capsule shaped tablet marked "Cardura" on one side, scored and marked "4 mg" on the other side.
8 mg Tablet
White, capsule shaped tablet with break score and marked "CN8" on one side and "Pfizer" on the other side.
Green, capsule shaped tablet marked "Cardura" on one side, scored and marked "8 mg" on the other side.
Active ingredient: doxazosin
Inactive ingredients: microcrystalline cellulose, lactose, sodium starch glycolate, magnesium stearate and sodium lauryl sulfate. The 2 mg yellow tablet contains D & C yellow 10 and FD & C yellow 6; the 4 mg orange tablet contains FD & C yellow 6; the 8 mg green tablet contains FD & C blue 2 and D & C yellow 10.

LAB-0070-6.0
For more information, go to www.Pfizer.com or call 1-800-438-1985.
- PRINCIPAL DISPLAY PANEL - 1 mg Tablet Bottle Label - 2750-66
- PRINCIPAL DISPLAY PANEL - 2 mg Tablet Bottle Label - 2760-66
- PRINCIPAL DISPLAY PANEL - 4 mg Tablet Bottle Label - 2770-66
- PRINCIPAL DISPLAY PANEL - 8 mg Tablet Bottle Label - 2780-66
- PRINCIPAL DISPLAY PANEL - 1 mg Tablet Bottle Label - 2410-10
- PRINCIPAL DISPLAY PANEL - 2 mg Tablet Bottle Label - 2512-10
- PRINCIPAL DISPLAY PANEL - 4 mg Tablet Bottle Label - 2614-10
- PRINCIPAL DISPLAY PANEL - 8 mg Tablet Bottle Label - 2716-10
-
INGREDIENTS AND APPEARANCE
CARDURA
doxazosin mesylate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0049-2750 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXAZOSIN MESYLATE (UNII: 86P6PQK0MU) (DOXAZOSIN - UNII:NW1291F1W8) DOXAZOSIN 1 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) MAGNESIUM STEARATE (UNII: 70097M6I30) SODIUM LAURYL SULFATE (UNII: 368GB5141J) Product Characteristics Color WHITE Score 2 pieces Shape OVAL Size 9mm Flavor Imprint Code Cardura;1;mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0049-2750-66 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/01/1991 01/31/2022 2 NDC:0049-2750-41 100 in 1 CARTON 11/01/1993 03/04/2003 2 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019668 01/01/1991 01/31/2022 CARDURA
doxazosin mesylate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0049-2760 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXAZOSIN MESYLATE (UNII: 86P6PQK0MU) (DOXAZOSIN - UNII:NW1291F1W8) DOXAZOSIN 2 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) MAGNESIUM STEARATE (UNII: 70097M6I30) SODIUM LAURYL SULFATE (UNII: 368GB5141J) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Product Characteristics Color YELLOW Score 2 pieces Shape OVAL Size 9mm Flavor Imprint Code Cardura;2;mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0049-2760-66 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/01/1991 04/30/2022 2 NDC:0049-2760-41 100 in 1 CARTON 11/01/1993 03/04/2003 2 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019668 01/01/1991 04/30/2022 CARDURA
doxazosin mesylate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0049-2770 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXAZOSIN MESYLATE (UNII: 86P6PQK0MU) (DOXAZOSIN - UNII:NW1291F1W8) DOXAZOSIN 4 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) MAGNESIUM STEARATE (UNII: 70097M6I30) SODIUM LAURYL SULFATE (UNII: 368GB5141J) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Product Characteristics Color ORANGE Score 2 pieces Shape OVAL Size 12mm Flavor Imprint Code Cardura;4;mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0049-2770-66 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/01/1991 04/30/2022 2 NDC:0049-2770-41 100 in 1 CARTON 11/01/1993 03/04/2003 2 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019668 01/01/1991 04/30/2022 CARDURA
doxazosin mesylate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0049-2780 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXAZOSIN MESYLATE (UNII: 86P6PQK0MU) (DOXAZOSIN - UNII:NW1291F1W8) DOXAZOSIN 8 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) MAGNESIUM STEARATE (UNII: 70097M6I30) SODIUM LAURYL SULFATE (UNII: 368GB5141J) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) Product Characteristics Color GREEN Score 2 pieces Shape OVAL Size 12mm Flavor Imprint Code Cardura;8;mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0049-2780-66 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/01/1991 09/30/2023 2 NDC:0049-2780-41 100 in 1 CARTON 11/01/1993 03/04/2003 2 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019668 01/01/1991 09/30/2023 CARDURA
doxazosin tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0049-2410 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXAZOSIN MESYLATE (UNII: 86P6PQK0MU) (DOXAZOSIN - UNII:NW1291F1W8) DOXAZOSIN 1 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) MAGNESIUM STEARATE (UNII: 70097M6I30) SODIUM LAURYL SULFATE (UNII: 368GB5141J) Product Characteristics Color WHITE Score no score Shape ROUND Size 7mm Flavor Imprint Code CN1;Pfizer Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0049-2410-10 100 in 1 BOTTLE; Type 0: Not a Combination Product 10/19/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019668 10/19/2021 CARDURA
doxazosin tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0049-2512 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXAZOSIN MESYLATE (UNII: 86P6PQK0MU) (DOXAZOSIN - UNII:NW1291F1W8) DOXAZOSIN 2 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) MAGNESIUM STEARATE (UNII: 70097M6I30) SODIUM LAURYL SULFATE (UNII: 368GB5141J) Product Characteristics Color WHITE Score 2 pieces Shape OVAL Size 9mm Flavor Imprint Code CN2;Pfizer Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0049-2512-10 100 in 1 BOTTLE; Type 0: Not a Combination Product 10/19/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019668 10/19/2021 CARDURA
doxazosin tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0049-2614 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXAZOSIN MESYLATE (UNII: 86P6PQK0MU) (DOXAZOSIN - UNII:NW1291F1W8) DOXAZOSIN 4 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) MAGNESIUM STEARATE (UNII: 70097M6I30) SODIUM LAURYL SULFATE (UNII: 368GB5141J) Product Characteristics Color WHITE Score 2 pieces Shape DIAMOND Size 12mm Flavor Imprint Code CN4;Pfizer Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0049-2614-10 100 in 1 BOTTLE; Type 0: Not a Combination Product 10/19/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019668 10/19/2021 CARDURA
doxazosin tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0049-2716 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXAZOSIN MESYLATE (UNII: 86P6PQK0MU) (DOXAZOSIN - UNII:NW1291F1W8) DOXAZOSIN 8 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) MAGNESIUM STEARATE (UNII: 70097M6I30) SODIUM LAURYL SULFATE (UNII: 368GB5141J) Product Characteristics Color WHITE Score 2 pieces Shape OVAL Size 12mm Flavor Imprint Code CN8;Pfizer Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0049-2716-10 100 in 1 BOTTLE; Type 0: Not a Combination Product 02/15/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019668 02/15/2023 Labeler - ROERIG (829076996)